For the following concentration cell, use the Nernst equation to determine the expected voltage. Also indicate the oxidation reaction, the reduction reaction, the cell reaction, and the overall expected EMF of the cell using the tables from powerpoint slides 11 and 12 and the Nernst equation. Once you have determined the cell reaction, write the conventional cell notation. Zn and Zn+2 (0.25M) mixed with Cu and Cu+2 (0.35M) **(Top screenshot is slide 11, and bottom is slide 12)**
For the following concentration cell, use the Nernst equation to determine the expected voltage. Also indicate the oxidation reaction, the reduction reaction, the cell reaction, and the overall expected EMF of the cell using the tables from powerpoint slides 11 and 12 and the Nernst equation. Once you have determined the cell reaction, write the conventional cell notation. Zn and Zn+2 (0.25M) mixed with Cu and Cu+2 (0.35M) **(Top screenshot is slide 11, and bottom is slide 12)**
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.25QAP
Related questions
Question
For the following concentration cell, use the Nernst equation to determine the expected voltage. Also indicate the
Zn and Zn+2 (0.25M) mixed with Cu and Cu+2 (0.35M)
**(Top screenshot is slide 11, and bottom is slide 12)**
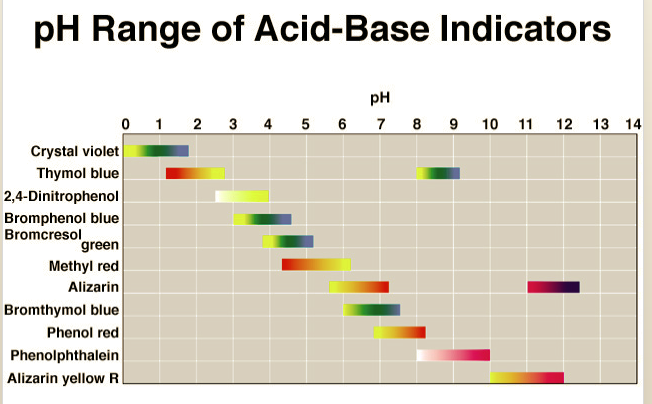
Transcribed Image Text:pH Range of Acid-Base Indicators
pH
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Crystal violet
Thymol blue
2,4-Dinitrophenol
Bromphenol blue
Bromcresol
green
Methyl red
Alizarin
Bromthymol blue
Phenol red
Phenolphthalein
Alizarin yellow R
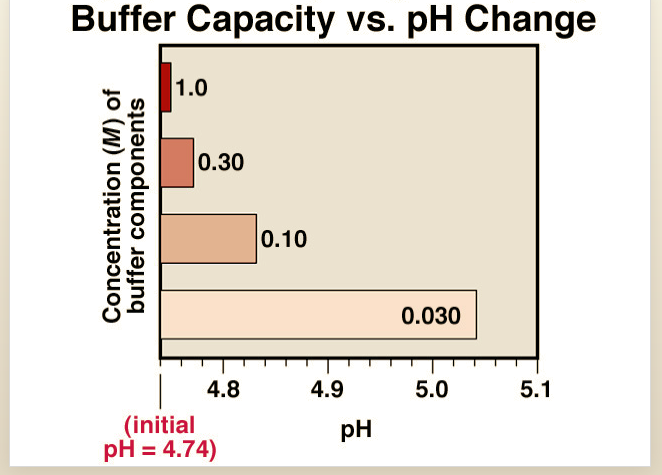
Transcribed Image Text:Buffer Capacity vs. pH Change
1.0
0.30
0.10
0.030
4.8
4.9
5.0
5.1
(initial
pH = 4.74)
pH
Concentration (M) of
buffer components
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



