600 500 400 Reactant 300 200 E, = 435 10 J 2 100 아 Initial state AE7X 10J -30 0° 30 60 90 120 150° 180 210 Reaction progress (angle of twist) Cis-2-butene is one of two forms of butene where the middle two carbon atoms are joined by a double bond. The other form is trans-2-butene. The conversion of the cis form to the trans form is slow at room temperature, and the two forms represent two distinct compounds. Conversion of the cis to the trans form involves rotation about the C-C double bond, which requires a substantial amount of energy on the molecular scale. Move the slider to observe the different conformations and their energies along the pathway of the conversion. Consider the linkage between the two central C atoms in cis-2-butene. How many sigma bonds join these two C atoms? Potential Energy 10 J
600 500 400 Reactant 300 200 E, = 435 10 J 2 100 아 Initial state AE7X 10J -30 0° 30 60 90 120 150° 180 210 Reaction progress (angle of twist) Cis-2-butene is one of two forms of butene where the middle two carbon atoms are joined by a double bond. The other form is trans-2-butene. The conversion of the cis form to the trans form is slow at room temperature, and the two forms represent two distinct compounds. Conversion of the cis to the trans form involves rotation about the C-C double bond, which requires a substantial amount of energy on the molecular scale. Move the slider to observe the different conformations and their energies along the pathway of the conversion. Consider the linkage between the two central C atoms in cis-2-butene. How many sigma bonds join these two C atoms? Potential Energy 10 J
Chapter3: Organic Compounds: Alkanes And Their Stereochemistry
Section3.SE: Something Extra
Problem 52AP: Increased substitution around a bond leads to increased strain. Take the four substituted butanes...
Related questions
Question
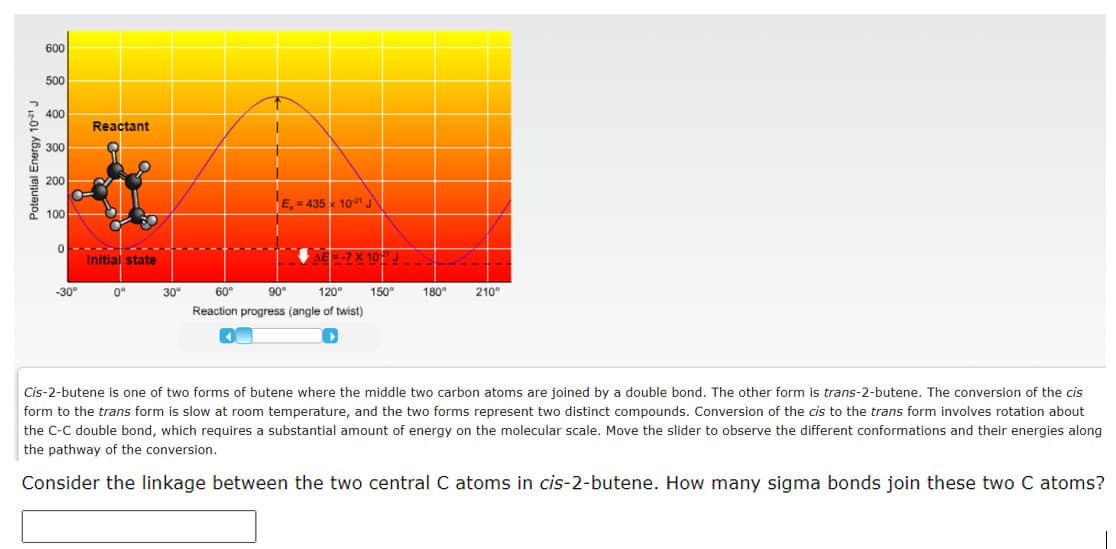
Transcribed Image Text:600
500
400
Reactant
300
画 200
E, = 435 10J
2 100
아--
Initial state
AE-7X 10 J
-30
0°
30
60
90
120
150°
180
210
Reaction progress (angle of twist)
Cis-2-butene is one of two forms of butene where the middle two carbon atoms are joined by a double bond. The other form is trans-2-butene. The conversion of the cis
form to the trans form is slow at room temperature, and the two forms represent two distinct compounds. Conversion of the cis to the trans form involves rotation about
the C-C double bond, which requires a substantial amount of energy on the molecular scale. Move the slider to observe the different conformations and their energies along
the pathway of the conversion.
Consider the linkage between the two central C atoms in cis-2-butene. How many sigma bonds join these two C atoms?
Potential Energy 10 J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning