How many milliliters of each solution did you use to make the your buffer? (please use three significant figures for your answer) 38.7 ml of acid: CH3COOH : and mL of base: NACH3C00 : 61.3 Your answer is incorrect, but you have 2 attempts left.
How many milliliters of each solution did you use to make the your buffer? (please use three significant figures for your answer) 38.7 ml of acid: CH3COOH : and mL of base: NACH3C00 : 61.3 Your answer is incorrect, but you have 2 attempts left.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 97AE: A student intends to titrate a solution of a weak monoprotic acid with a sodium hydroxide solution...
Related questions
Question
drop down for the first selection is NH4Cl and CH3COOH and the second drop down is NH3 and NaCH3COOH
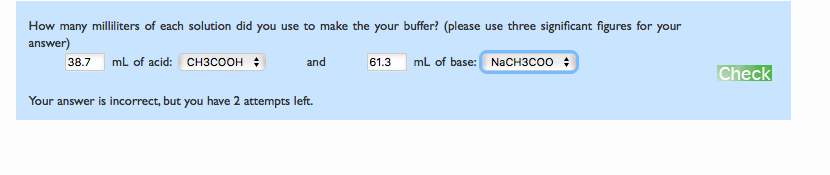
Transcribed Image Text:How many milliliters of each solution did you use to make the your buffer? (please use three significant figures for your
answer)
mL of acid: Cнзсоон н
mL of base: NaCH3C00 :
38.7
and
61.3
Check
Your answer is incorrect, but you have 2 attempts left.

Transcribed Image Text:In this activity you will be using the virtual lab to create 100mL of a buffer solution that has a pH of 4.58M
The virtual lab stockroom has a number of 0.IM weak acids and conjugate bases to help you in this process. When you have completed
making your buffer solution, use the form at the bottom of the page to check your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning