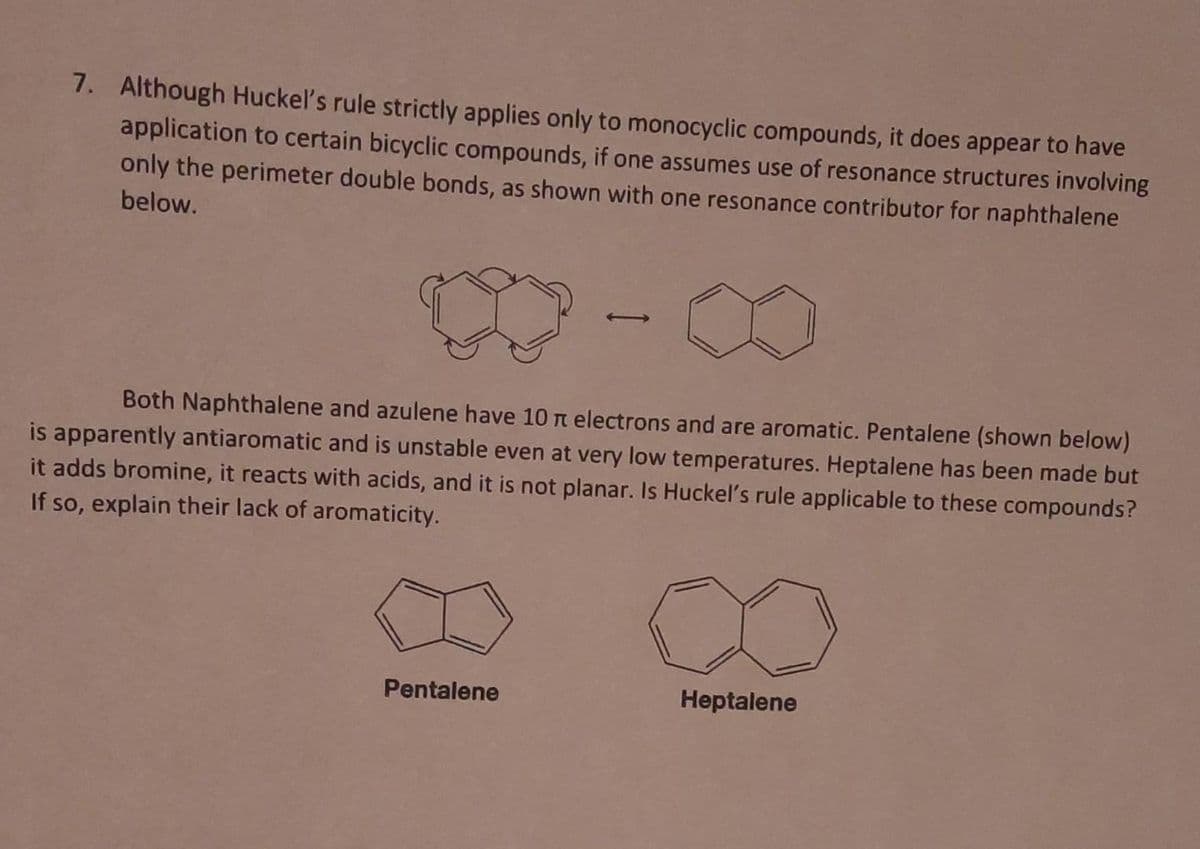
7. Although Huckel's rule strictly applies only to monocyclic compounds, it does appear to have application to certain bicyclic compounds, if one assumes use of resonance structures involving only the perimeter double bonds, as shown with one resonance contributor for naphthalene below. Both Naphthalene and azulene have 10 n electrons and are aromatic. Pentalene (shown below) is apparently antiaromatic and is unstable even at very low temperatures. Heptalene has been made but it adds bromine, it reacts with acids, and it is not planar. Is Huckel's rule applicable to these compounds? If so, explain their lack of aromaticity. Pentalene Heptalene
Reactions of Ethers
Ethers (R-O-R’) are compounds formed by replacing hydrogen atoms of an alcohol (R-OH compound) or a phenol (C6H5OH) by an aryl/ acyl group (functional group after removing single hydrogen from an aromatic ring). In this section, reaction, preparation and behavior of ethers are discussed in the context of organic chemistry.
Epoxides
Epoxides are a special class of cyclic ethers which are an important functional group in organic chemistry and generate reactive centers due to their unusual high reactivity. Due to their high reactivity, epoxides are considered to be toxic and mutagenic.
Williamson Ether Synthesis
An organic reaction in which an organohalide and a deprotonated alcohol forms ether is known as Williamson ether synthesis. Alexander Williamson developed the Williamson ether synthesis in 1850. The formation of ether in this synthesis is an SN2 reaction.
I need help ASAP...please help.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images









