7. Assume that each of the following atoms reacts with ,hydrogen: (i) boron; (ii) silicon; (iii) nitrogen; and (iv) chlorine. TA C (a) Write the electron configuration for each atom. (b) Draw the orbital diagrams, showing the valence electrons. (c) Determine whether any electron needs to be promoted. If so, draw a new orbital diagram showing the excited-state atom. Chapter 4 • Chemical Bonding
7. Assume that each of the following atoms reacts with ,hydrogen: (i) boron; (ii) silicon; (iii) nitrogen; and (iv) chlorine. TA C (a) Write the electron configuration for each atom. (b) Draw the orbital diagrams, showing the valence electrons. (c) Determine whether any electron needs to be promoted. If so, draw a new orbital diagram showing the excited-state atom. Chapter 4 • Chemical Bonding
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter20: Organic Chemistry
Section: Chapter Questions
Problem 4A
Related questions
Question
Solve all parts otherwise I will downvote

Transcribed Image Text:7. Assume that each of the following atoms reacts with
,hydrogen: (i) boron; (ii) silicon; (iii) nitrogen; and
(iv) chlorine. u TA C
(a) Write the electron configuration for each atom.
(b) Draw the orbital diagrams, showing the valence
electrons.
(c) Determine whether any electron needs to be
promoted. If so, draw a new orbital diagram
showing the excited-state atom.
Chapter 4 Chemical Bonding
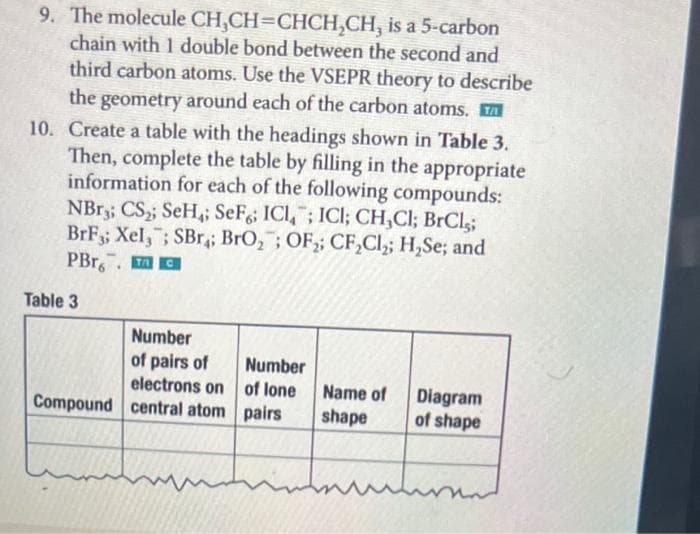
Transcribed Image Text:9. The molecule CH,CH=CHCH,CH, is a 5-carbon
chain with 1 double bond between the second and
third carbon atoms. Use the VSEPR theory to describe
the geometry around each of the carbon atoms.
10. Create a table with the headings shown in Table 3.
Then, complete the table by filling in the appropriate
information for each of the following compounds:
NBR3; CS; SeH,; SeF; ICI,; ICI; CH,Cl; BrCl,;
BrF3; Xel,; SBr; BrO,; OF,; CF,Cl,; H,Se; and
PBr. O
Table 3
Number
of pairs of
electrons on
Compound central atom pairs
Number
of lone
Name of
Diagram
of shape
shape
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning