7. Consider the following balanced thermochemical equationfor a reaction sometimes use for H2S production: %S8(s) + H2(g) → H2S(g) AHrxn = -20.2 kJ (a) Is this an exothermic or endothermic reaction? (b) What is AHrxn for the reverse reaction? (c) What is AH when 3.2 mol of Sa reacts? (d) What is AH when 20.0 g of S8 reacts?
7. Consider the following balanced thermochemical equationfor a reaction sometimes use for H2S production: %S8(s) + H2(g) → H2S(g) AHrxn = -20.2 kJ (a) Is this an exothermic or endothermic reaction? (b) What is AHrxn for the reverse reaction? (c) What is AH when 3.2 mol of Sa reacts? (d) What is AH when 20.0 g of S8 reacts?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.132QP
Related questions
Question
100%
hello maam/sir kindly help me with this one.. pleaseeee... kindly show solution also please... thanks in advance
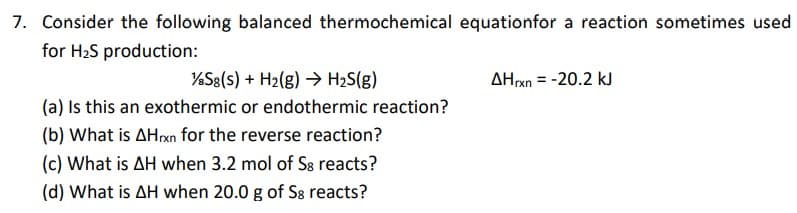
Transcribed Image Text:7. Consider the following balanced thermochemical equationfor a reaction sometimes used
for H2S production:
%S8(s) + H2(g) → H2S(g)
(a) Is this an exothermic or endothermic reaction?
AHxn = -20.2 kJ
(b) What is AHrxn for the reverse reaction?
(c) What is AH when 3.2 mol of S8 reacts?
(d) What is AH when 20.0 g of S8 reacts?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning