Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.3E
Related questions
Question
Using Figure 12.4 predict the expected boiling point and mole percentage of pentane
expected if a solution which was 20 % pentane and 80% hexane (mol percent) was distilled
(only one equilibrium temperature/theoretical plate)?
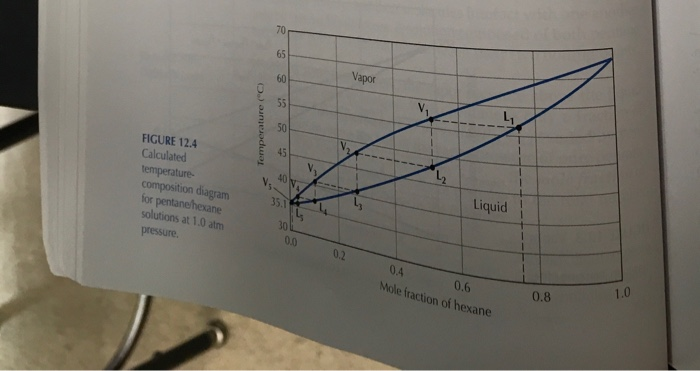
Transcribed Image Text:70
65
Vapor
60
55
50
FIGURE 12.4
Calculated
45
40 y
temperature-
composition diagram
for pentanehexane
solutions at 1.0 atm
Liquid
35.1
30
0.0
pressure.
0.2
0.4
1.0
0.6
Mole fraction of hexane
0.8
Temperature ("C)
Expert Solution
Step 1t
According to the plot given 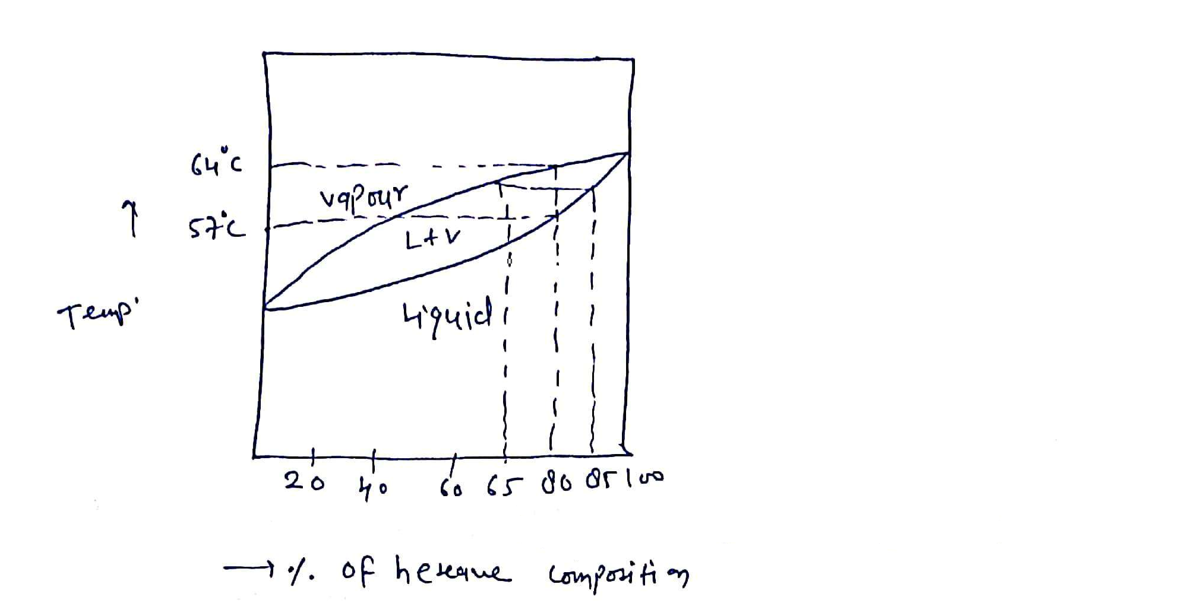
From the plot it is clear that phase transition takes place. At C, the phase transition from L + V to vapor phase occurs for the 80% hexane and 20% pentane.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
