70 LABORATORY REPORT Chemistry of Cells d. A molecule formed by an ionic bond is shown below. Using Table 2, indicate the atoms involved, the chemical formula of the molecule, and the number of electrons transfered. Draw arrows to show the direction of electron transfer. 17p 18n omado in 20m 17p 180 u bi lo noteng lo dmun Atoms involved Chemical formula Number of electrons transfered e Ising Table ? and vour aneTuer to itonm ?d nhoun
70 LABORATORY REPORT Chemistry of Cells d. A molecule formed by an ionic bond is shown below. Using Table 2, indicate the atoms involved, the chemical formula of the molecule, and the number of electrons transfered. Draw arrows to show the direction of electron transfer. 17p 18n omado in 20m 17p 180 u bi lo noteng lo dmun Atoms involved Chemical formula Number of electrons transfered e Ising Table ? and vour aneTuer to itonm ?d nhoun
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 55E: Glucose is the major fuel for most living cells. The oxidative breakdown of glucose by our body to...
Related questions
Question
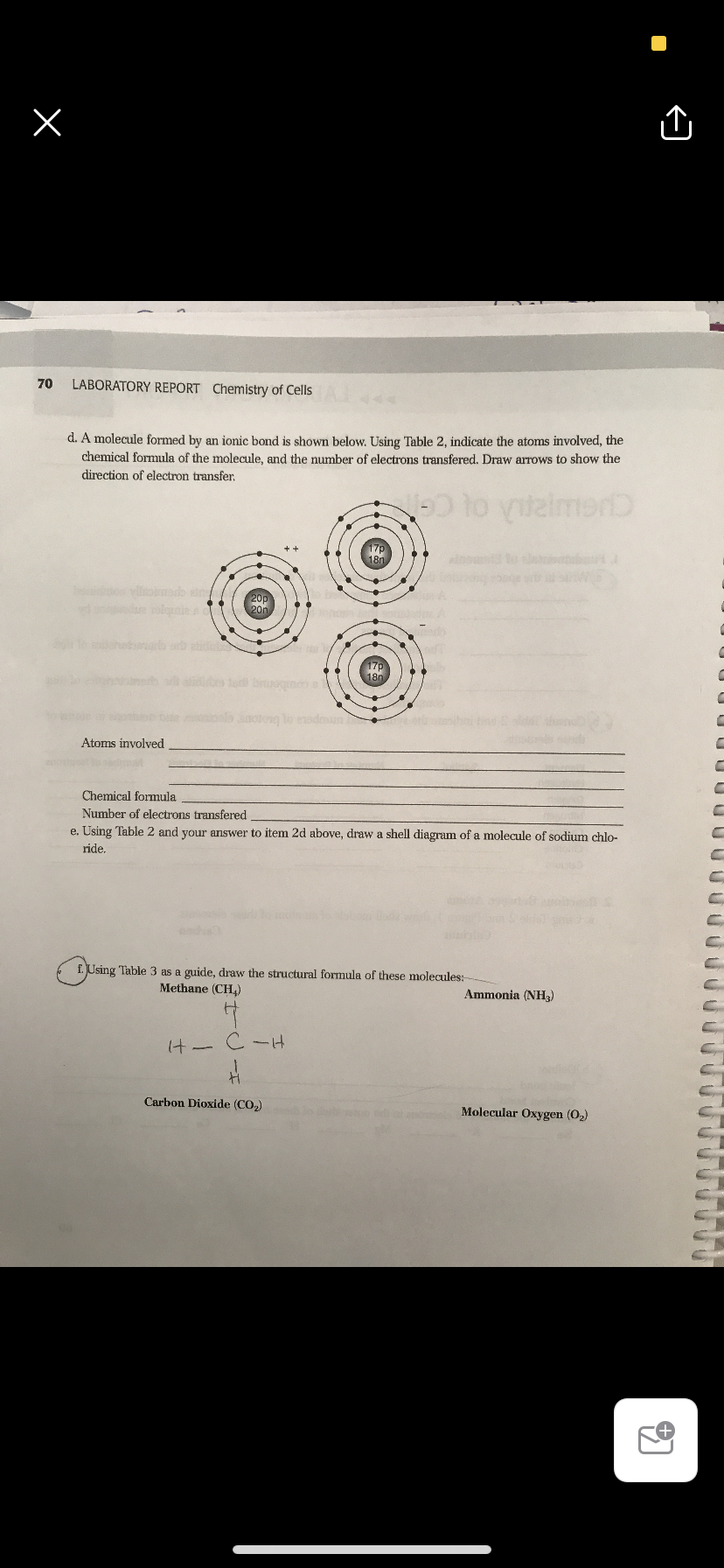
Transcribed Image Text:70
LABORATORY REPORT Chemistry of Cells
d. A molecule formed by an ionic bond is shown below. Using Table 2, indicate the atoms involved, the
chemical formula of the molecule, and the number of electrons transfered. Draw arrows to show the
direction of electron transfer,
aloo to sletb
ahe b rb di
s t bgo a
e bie lnoteng lo enadmn
ld
Atoms involved
Chemical formula
Number of electrons transfered
e. Using Table 2 and your answer to item 2d above, draw a shell diagram of a molecule of sodium chlo-
ride.
f. Using Table 3 as a guide, draw the structural formula of these molecules:
Methane (CH,)
Ammonia (NH,)
けー
C-H
Carbon Dioxide (CO2)
Molecular Oxygen (O2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole
