7:45 PM Wed Dec 4 Done www-awu.aleks.com AA O MEASUREMENT Multiplication and division of measurements Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write Is this proposed multiplication or division result x10 possible? O yes (1.0 ke) - (s0 m²) - ? 2 (1.0 kg) - (5.0 m %3D no O yes 360. ms = ? O no 0.090 s O yes (2.0 mg) - (0.028 g) = ? %D no Check Explanation 2019 McGraw-Hill Education.
7:45 PM Wed Dec 4 Done www-awu.aleks.com AA O MEASUREMENT Multiplication and division of measurements Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write Is this proposed multiplication or division result x10 possible? O yes (1.0 ke) - (s0 m²) - ? 2 (1.0 kg) - (5.0 m %3D no O yes 360. ms = ? O no 0.090 s O yes (2.0 mg) - (0.028 g) = ? %D no Check Explanation 2019 McGraw-Hill Education.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
How can I solve these in an easy manner?
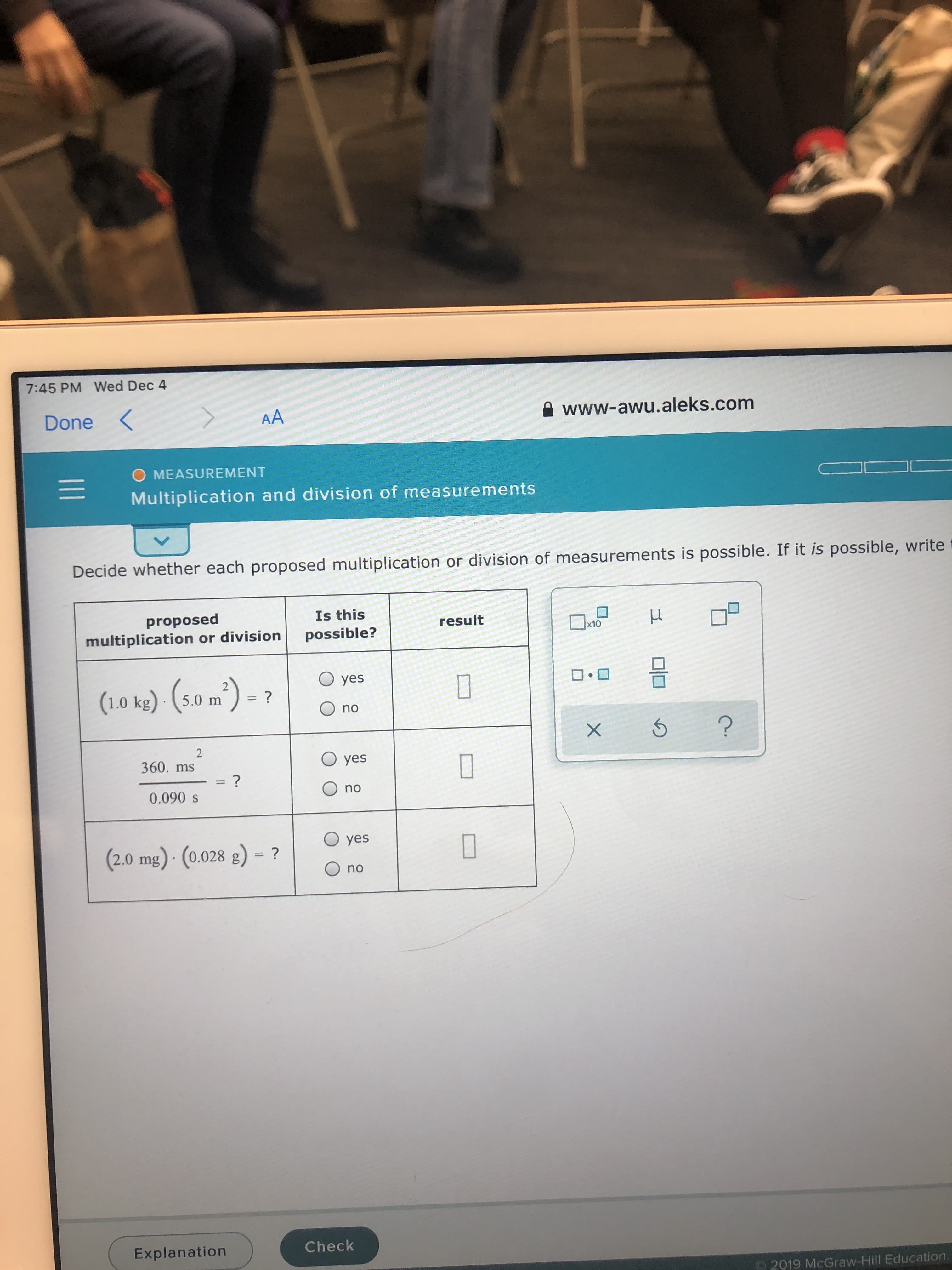
Transcribed Image Text:7:45 PM Wed Dec 4
Done
www-awu.aleks.com
AA
O MEASUREMENT
Multiplication and division of measurements
Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write
Is this
proposed
multiplication or division
result
x10
possible?
O yes
(1.0 ke) - (s0 m²) - ?
2
(1.0 kg) - (5.0 m
%3D
no
O yes
360. ms
= ?
O no
0.090 s
O yes
(2.0 mg) - (0.028 g) = ?
%D
no
Check
Explanation
2019 McGraw-Hill Education.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
