8) Consider the heteronuclear diatomic molecule, HCl with bonding orbital given by, W, = 0.200,H +0.989,,, C where is the (normalized) hydrogen 1s orbital and , a is the (normalized) chlorine 3pz orbital. Assume that the atomic overlap integral is zero, YW3P.cdt =0. all space a) Show that the bonding molecular orbital is normalized. b) What is the energy of the bonding orbital? Write your answer in terms of a,,a,, and B, which are defined by: S Wop,ci Hwdr = ß all space all space %3D | Vis. Hyudt = a, and ] Wap.c Hwap.cd7 = d;p. %3D | V1s,H all space all space
8) Consider the heteronuclear diatomic molecule, HCl with bonding orbital given by, W, = 0.200,H +0.989,,, C where is the (normalized) hydrogen 1s orbital and , a is the (normalized) chlorine 3pz orbital. Assume that the atomic overlap integral is zero, YW3P.cdt =0. all space a) Show that the bonding molecular orbital is normalized. b) What is the energy of the bonding orbital? Write your answer in terms of a,,a,, and B, which are defined by: S Wop,ci Hwdr = ß all space all space %3D | Vis. Hyudt = a, and ] Wap.c Hwap.cd7 = d;p. %3D | V1s,H all space all space
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section: Chapter Questions
Problem 19PS: Considering only the molecular orbitals formed by combinations of the 2s atomic orbitals, how many...
Related questions
Question
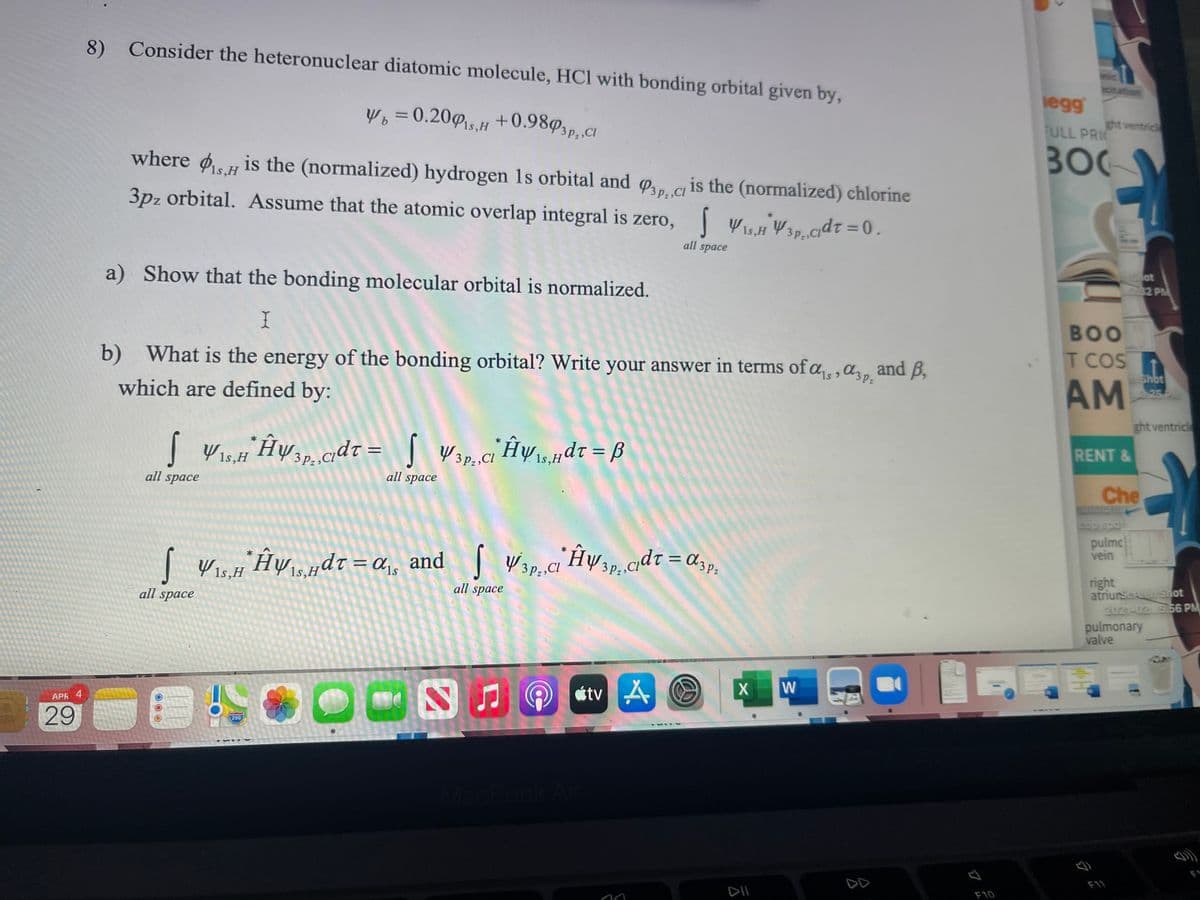
Transcribed Image Text:8) Consider the heteronuclear diatomic molecule, HCl with bonding orbital given by,
citation
legg
W, = 0.205,H +0.98º3p,cI
%D
ght ventric
FULL PRIC
where ø is the (normalized) hydrogen 1s orbital and o,, c is the (normalized) chlorine
BOG
3pz orbital. Assume that the atomic overlap integral is zero, | WisH W3p.cadt = 0.
%3D
all space
a) Show that the bonding molecular orbital is normalized.
Shot
232 PM
BOO
and B,
b) What is the energy of the bonding orbital? Write your answer in terms of a,, ,a,,.
T COS
which are defined by:
АМ
Shbt
25PM
ght ventric
| V1s,H
Hw cdt = |
4 1s,H
RENT &
||
all space
all space
Che
pulmc
vein
| Wis.H HySHdt =a,, and
cdt = d3p.
1s,
3 Pz,CI
right
atriursen Shot
2021-02 5.56 PM
pulmonary
valve
all space
all space
W
étv A
APR 4
29
260
Book A
DD
F11
F10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning