Assume that each H-bond is formed by two dipoles in a line, and approximate each dipole as a pair of point charges (q1, -q1, q2, -q2) separated by a distance r1=r2 with the centers of the dipoles at distance D apart. Then, the potential energy of the collinear arrangement of two dipoles is U=2µ1 H2/(4tE,D³). Assuming that each H-bond has an energy of 10KJ/mol, and that the charges q1 and q2 are equivalent, use the equation for the potential energy of a collinear arrangement of two dipoles to estimate the moment of the dipoles (µ1 = µ2) involved. Now, assuming that the charge separation distance in each dipole is 1 angstrom, estimate the magnitude of the partial charges responsible for the H-bond (report your answer in units of elementary charge, not coulombs).
Assume that each H-bond is formed by two dipoles in a line, and approximate each dipole as a pair of point charges (q1, -q1, q2, -q2) separated by a distance r1=r2 with the centers of the dipoles at distance D apart. Then, the potential energy of the collinear arrangement of two dipoles is U=2µ1 H2/(4tE,D³). Assuming that each H-bond has an energy of 10KJ/mol, and that the charges q1 and q2 are equivalent, use the equation for the potential energy of a collinear arrangement of two dipoles to estimate the moment of the dipoles (µ1 = µ2) involved. Now, assuming that the charge separation distance in each dipole is 1 angstrom, estimate the magnitude of the partial charges responsible for the H-bond (report your answer in units of elementary charge, not coulombs).
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 10CTQ
Related questions
Question
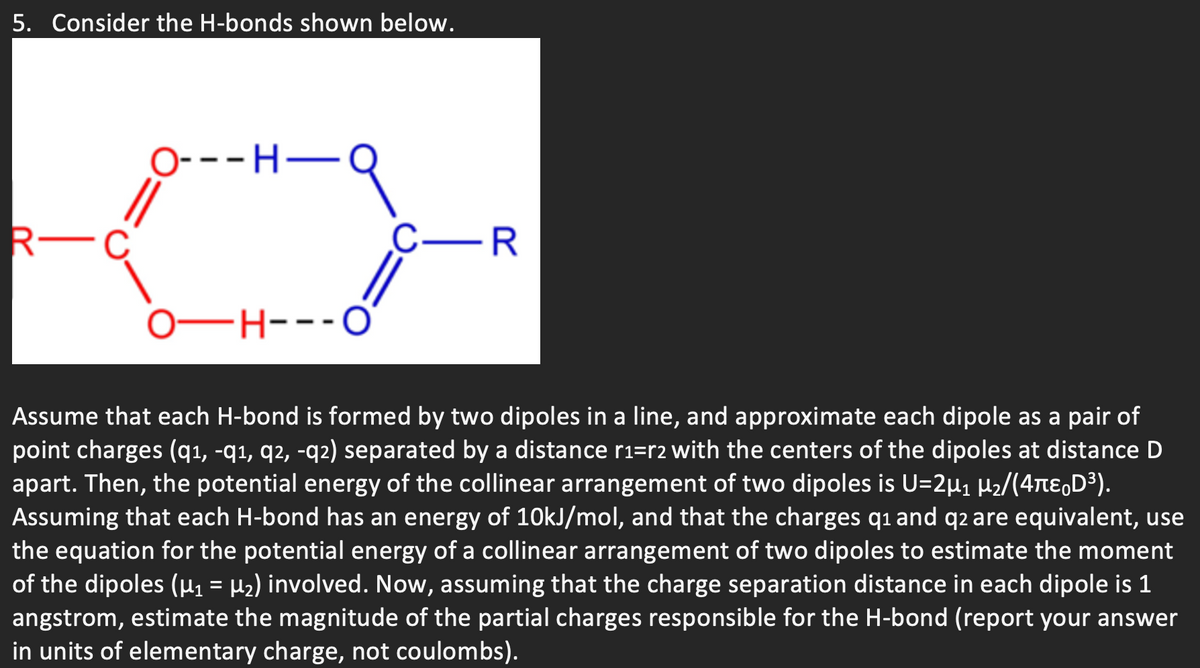
Transcribed Image Text:5. Consider the H-bonds shown below.
O---H-
R-
C-R
0–H--
FH---O'
Assume that each H-bond is formed by two dipoles in a line, and approximate each dipole as a pair of
point charges (q1, -q1, q2, -q2) separated by a distance r1=r2 with the centers of the dipoles at distance D
apart. Then, the potential energy of the collinear arrangement of two dipoles is U=2µ1 H2/(4te,D³).
Assuming that each H-bond has an energy of 1OKJ/mol, and that the charges q1 and q2 are equivalent, use
the equation for the potential energy of a collinear arrangement of two dipoles to estimate the moment
of the dipoles (µ1 = µ2) involved. Now, assuming that the charge separation distance in each dipole is 1
angstrom, estimate the magnitude of the partial charges responsible for the H-bond (report your answer
in units of elementary charge, not coulombs).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning