8) If the heat of fusion for water is 80. cal/g, how many calories are needed to melt 45.0 g of ice at 0 °C? A) 0.56 cal B) 80. cal C) 3.6 cal D) 1.8 cal 81 Insimal olgmootesd ir moils ono nimuis E) 3.6 x 103 cal os6.0 (8
8) If the heat of fusion for water is 80. cal/g, how many calories are needed to melt 45.0 g of ice at 0 °C? A) 0.56 cal B) 80. cal C) 3.6 cal D) 1.8 cal 81 Insimal olgmootesd ir moils ono nimuis E) 3.6 x 103 cal os6.0 (8
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem 8RE
Related questions
Question
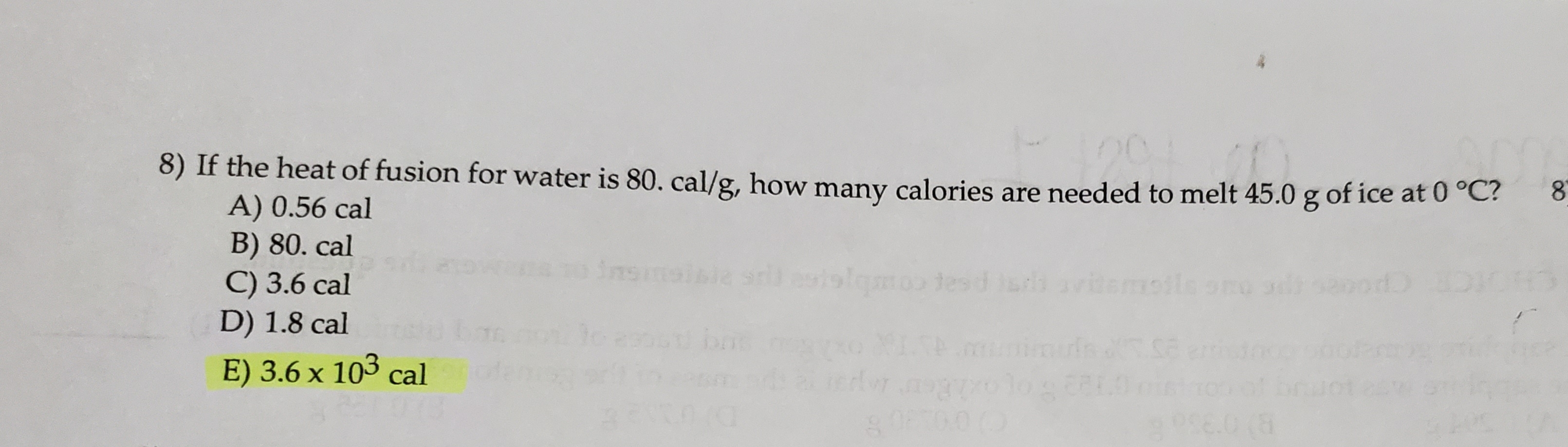
Transcribed Image Text:8) If the heat of fusion for water is 80. cal/g, how many calories are needed to melt 45.0 g of ice at 0 °C?
A) 0.56 cal
B) 80. cal
C) 3.6 cal
D) 1.8 cal
81
Insimal
olgmootesd ir
moils ono
nimuis
E) 3.6 x 103 cal
os6.0 (8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
