8 of 2 I Review | Constants | Periodic T Part A Suppose that, from measurements in a microscope, you determine that a certain layer of graphene covers an area of 2.60 um?. Convert this to square meters. 2. Express the area in square meters to three significant figures. • View Available Hint(s) ΑΣφ 2.60 µm? Submit P Pearson on Inc. All rights reserved. I Terms of Use | Privacy Policy I Permissions Contact Us 4:39 PM 25% 1/31/2020
8 of 2 I Review | Constants | Periodic T Part A Suppose that, from measurements in a microscope, you determine that a certain layer of graphene covers an area of 2.60 um?. Convert this to square meters. 2. Express the area in square meters to three significant figures. • View Available Hint(s) ΑΣφ 2.60 µm? Submit P Pearson on Inc. All rights reserved. I Terms of Use | Privacy Policy I Permissions Contact Us 4:39 PM 25% 1/31/2020
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
ChapterA: Scientific Notation And Experimental Error
Section: Chapter Questions
Problem 8P
Related questions
Question
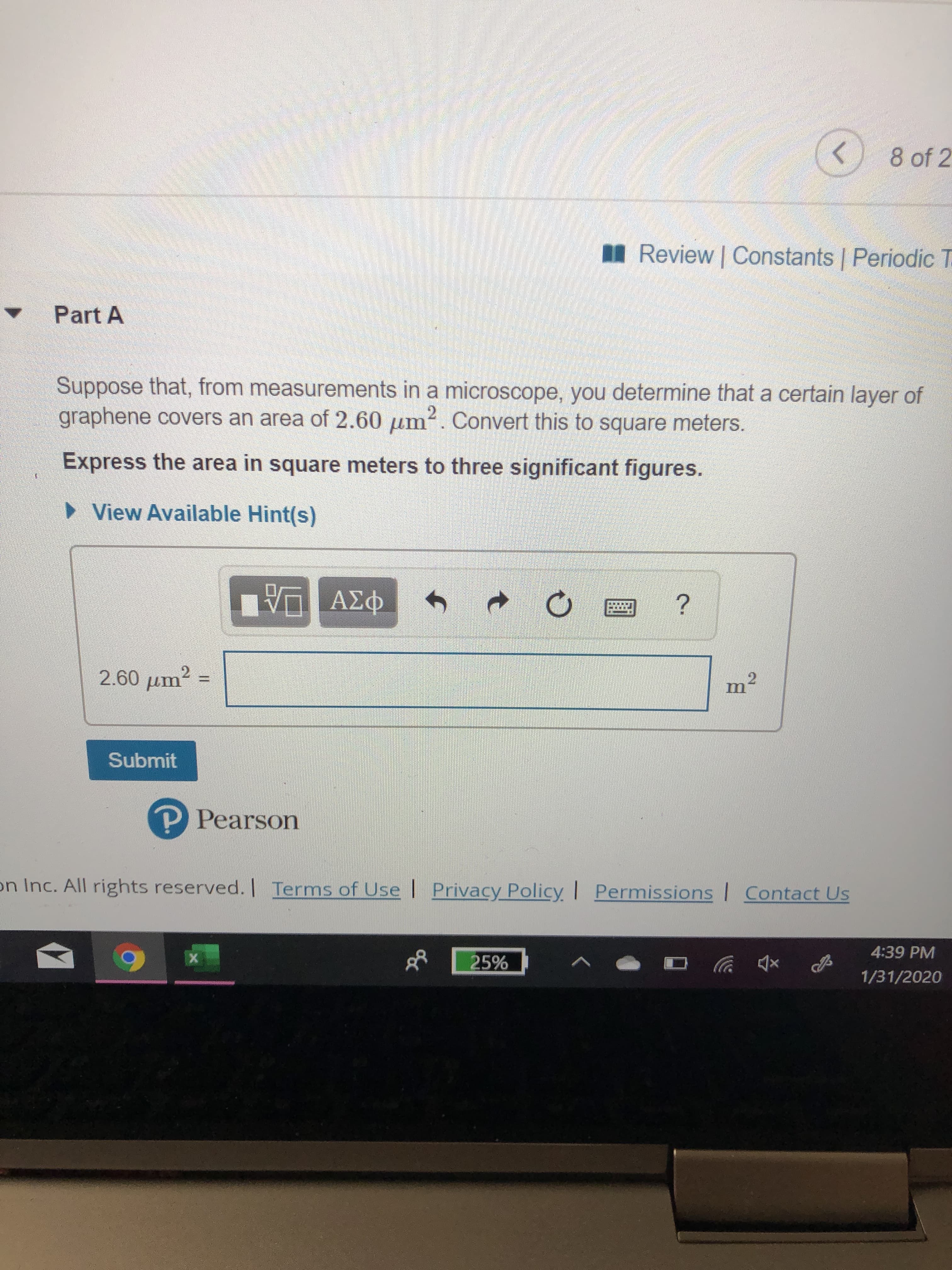
Transcribed Image Text:8 of 2
I Review | Constants | Periodic T
Part A
Suppose that, from measurements in a microscope, you determine that a certain layer of
graphene covers an area of 2.60 um?. Convert this to square meters.
2.
Express the area in square meters to three significant figures.
• View Available Hint(s)
ΑΣφ
2.60 µm?
Submit
P Pearson
on Inc. All rights reserved. I Terms of Use | Privacy Policy I Permissions
Contact Us
4:39 PM
25%
1/31/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning