8. Based on the observations above, does a chemical reaction take place When you MIx thnese substances? Yes. Because it turns to liquid. the temperature goes down. Also the smell changes. a. Which of these observations would you use to serve as data to support your claim? b. Do you think a process like this could be used as energy for moving a car? 9. Please insert the class consensus model of a car engine at the particle level. OXygen 一
8. Based on the observations above, does a chemical reaction take place When you MIx thnese substances? Yes. Because it turns to liquid. the temperature goes down. Also the smell changes. a. Which of these observations would you use to serve as data to support your claim? b. Do you think a process like this could be used as energy for moving a car? 9. Please insert the class consensus model of a car engine at the particle level. OXygen 一
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 13PS
Related questions
Question
Please clear handwriting… answer letter a and b
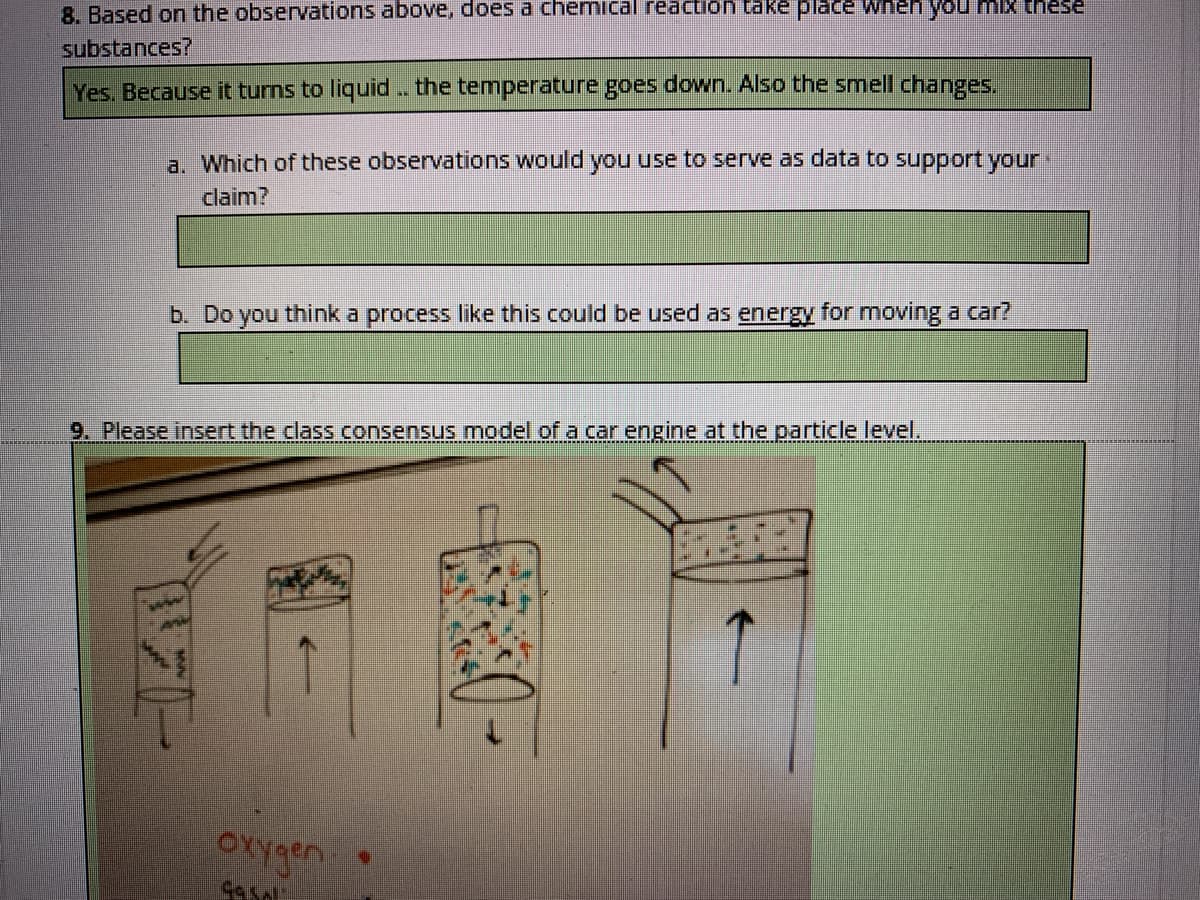
Transcribed Image Text:8. Based on the observations above, does a chemnical reaction take place Wneh ou mi these
substances?
Yes. Because it turns to liquid. the temperature goes down. Also the smell changes.
a. Which of these observations would yoU use to serve as data to support your
claim?
b. Do you think a process like this could be used as energy for moving a car?
9. Please insert the class consensus model of a car engine at the particle level.
OXygen

Transcribed Image Text:Answer Question 8
→ Do you think this was a chemical reaction and what observations
support your idea?
What does that mean about energy transfer?
→ Do you think a reaction like this could power a car?
→ Did this raise any questions for you?
→ If we were to model this process using our LOL diagrams what might
that look like?
→ It seems like a chemical reaction due to the presence of a new gos (evidence:
the very bad smell that wasn't there before)
→ The mixture looked like it chonged, we think we hove a liquid that wasn't
present before.
→ Both the barium hydroxide and ammonium chloride storted out ot room
temperature, but the surroundings got really cold - moybe the reaction is
getting energy transferred to it from the environment.
→ We are starting to think that temperature change in ony direction can be an
indication of a chemical reaction
→ Maybe if we can apply o reaction to our model we con figure out if it can make
our cor go.
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning