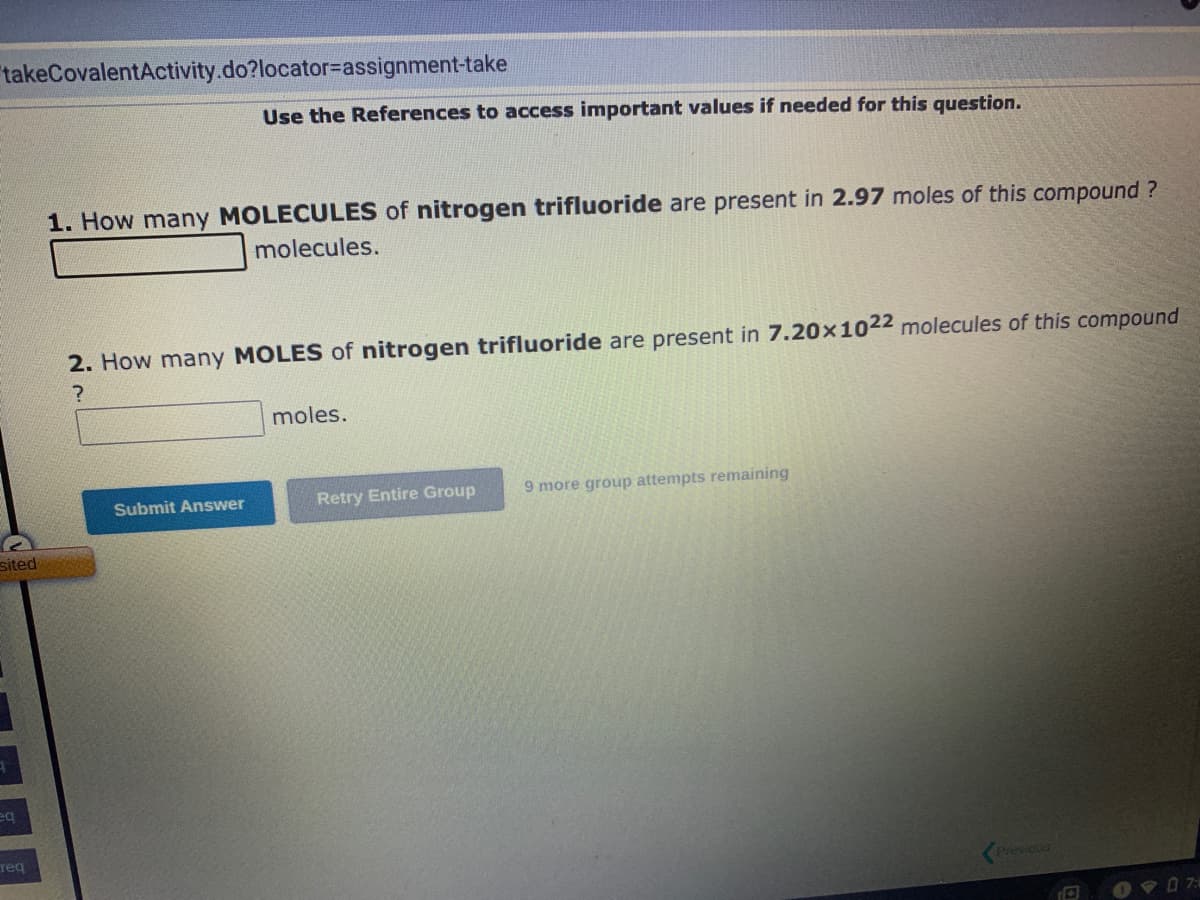
1. How many MOLECULES of nitrogen trifluoride are present in 2.97 moles of this compound? molecules. 2. How many MOLES of nitrogen trifluoride are present in 7.20×1022 molecules of this compound ? moles.
1. How many MOLECULES of nitrogen trifluoride are present in 2.97 moles of this compound? molecules. 2. How many MOLES of nitrogen trifluoride are present in 7.20×1022 molecules of this compound ? moles.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
Transcribed Image Text:takeCovalentActivity.do?locator-assignment-take
sited
eq
Teq
Use the References to access important values if needed for this question.
1. How many MOLECULES of nitrogen trifluoride are present in 2.97 moles of this compound?
molecules.
2. How many MOLES of nitrogen trifluoride are present in 7.20 x 1022 molecules of this compound
?
Submit Answer
moles.
Retry Entire Group
9 more group attempts remaining
Previous
07:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
