8.2x10 15 Accuracy and Precision Exercise A student uses 4 different instruments to measure 1ml of water. The density of water is approximately 1g/ml (it varies based on temperature) so 1ml of water should weigh 1g. 1. Complete the average mass and % error column in the chart. In your percent error calculations, use the average mass as your experimental value and 1g as your theoretical value. % error should be rounded to 2 decimal places. (8 points) Trial 1 Trial 2 Trial 3 Average % Error Mass of 1ml of 1.212 water measured with a "baby 1.254g 0.857g 1.524g beaker" Mass of 1ml of water measured with graduated cylinder Mass of 1ml of 0.958g 1.12g 0.915g water measured 0.998g 0.999g 0.999g with Micropipette #1 Mass of 1ml of water measured 1.251g 1.250g 1.251g with Micropipette #2 1. Comment on the relative accuracy/precision of each of the 4 instruments in the table below. yes or no will suffice) (8 points) Accurate? Precise?
8.2x10 15 Accuracy and Precision Exercise A student uses 4 different instruments to measure 1ml of water. The density of water is approximately 1g/ml (it varies based on temperature) so 1ml of water should weigh 1g. 1. Complete the average mass and % error column in the chart. In your percent error calculations, use the average mass as your experimental value and 1g as your theoretical value. % error should be rounded to 2 decimal places. (8 points) Trial 1 Trial 2 Trial 3 Average % Error Mass of 1ml of 1.212 water measured with a "baby 1.254g 0.857g 1.524g beaker" Mass of 1ml of water measured with graduated cylinder Mass of 1ml of 0.958g 1.12g 0.915g water measured 0.998g 0.999g 0.999g with Micropipette #1 Mass of 1ml of water measured 1.251g 1.250g 1.251g with Micropipette #2 1. Comment on the relative accuracy/precision of each of the 4 instruments in the table below. yes or no will suffice) (8 points) Accurate? Precise?
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.17QAP
Related questions
Question
100%
How do I fill the tables out?
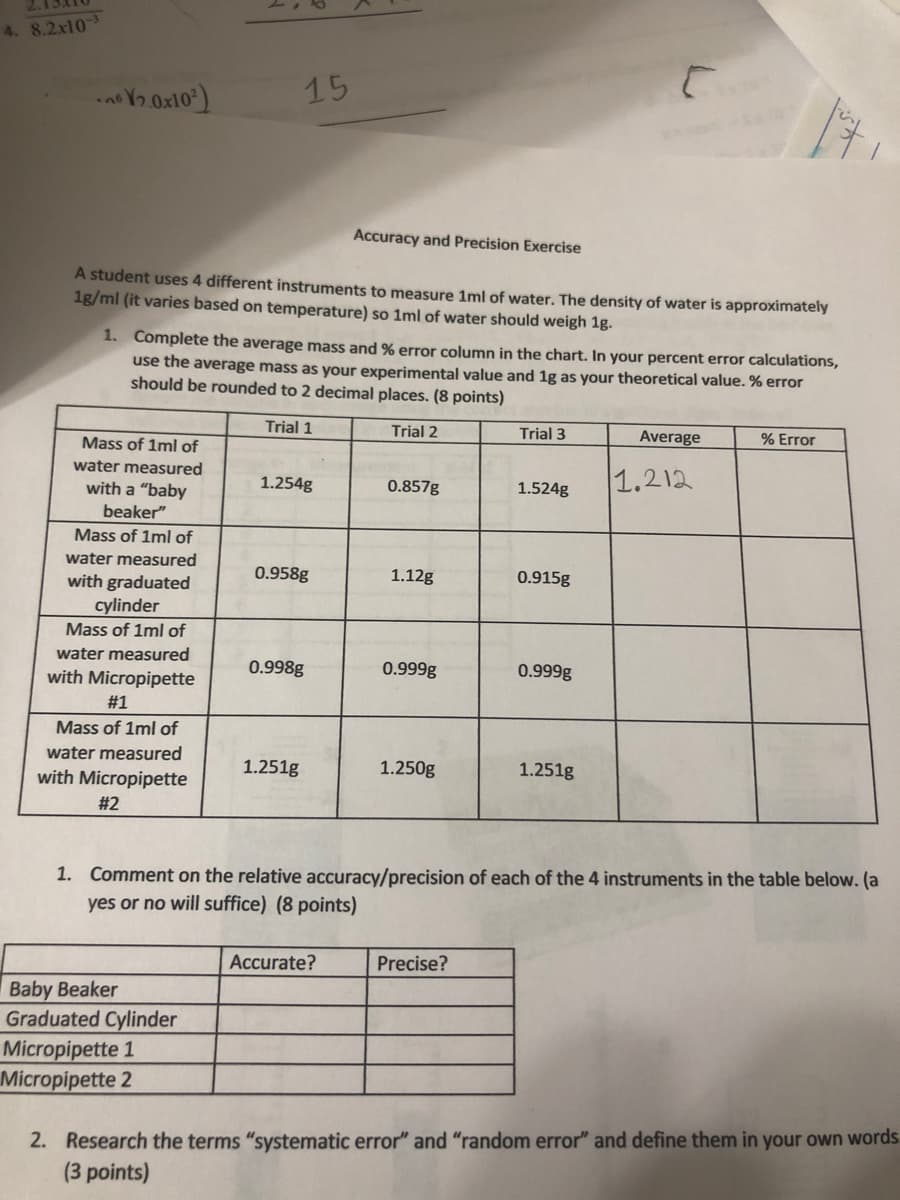
Transcribed Image Text:15
Accuracy and Precision Exercise
A student uses 4 different instruments to measure 1ml of water. The density of water is approximately
1g/ml (it varies based on temperature) so 1ml of water should weigh 1g.
1. Complete the average mass and % error column in the chart. In your percent error calculations,
use the average mass as your experimental value and 1g as your theoretical value. % error
should be rounded to 2 decimal places. (8 points)
Trial 1
Trial 2
Trial 3
Average
% Error
Mass of 1ml of
1.212
water measured
1.254g
0.857g
1.524g
with a "baby
beaker"
Mass of 1ml of
water measured
with graduated
cylinder
Mass of 1ml of
water measured
vith Micropipette
0.958g
1.12g
0.915g
0.998g
0.999g
0.999g
#1
Mass of 1ml of
water measured
th Micropipette
1.251g
1.250g
1.251g
#2
1. Comment on the relative accuracy/precision of each of the 4 instruments in the table below. (a
yes or no will suffice) (8 points)
Accurate?
Precise?
Beaker
ated Cylinder
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning