87. Tetraammine dicyano vanadium(1) 1. is a neutral complex 2. can exhibil geometric isomerism 3 is an octahedral complex 95. 88. Which of the following ligands can serve in linkage isomerism 1. C N:] 96. C 2. S C-N: 3. :0-N-O 89. Complex ions formed by d-block elements may be 1. cationic 2. neutral 3. anionic
87. Tetraammine dicyano vanadium(1) 1. is a neutral complex 2. can exhibil geometric isomerism 3 is an octahedral complex 95. 88. Which of the following ligands can serve in linkage isomerism 1. C N:] 96. C 2. S C-N: 3. :0-N-O 89. Complex ions formed by d-block elements may be 1. cationic 2. neutral 3. anionic
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.30QAP
Related questions
Question
Answer Q87, 88, 89 showing detailed explanations.
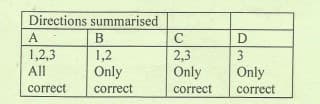
Transcribed Image Text:Directions summarised
A
B
1,2,3
1,2
2,3
Only
All
Only
Only
correct
correct
correct
correct
![o Scandium and Zinc are not consideren
transition elements because they
1 do not form complexes
2 exist only in one oxidation state
do not form ions in which the 34.
84 The olements in between Sc-Zn
1.
all show paramagnetism
2 form ocloured hydrated ions in aqueous
solution
3.
3. form ions that hive incompletely filled
3d-subshell.
subshell is partially filled.
92 d-biock element
85 This question relates to compounds of
chromium
1.
have variable oxidation state
1.
their +2 oxidation state is common at
2.
the beginning of the series.
3. form complex ions
chromium can form chormate(VII) and
dichromate (Vil) compounds which are
respectively orange and yellow in
colour
2.
In order to form a complex ion
the central metal ion or atom shoul
93.
the dichromate compound is made by
aciditying solutions of the chromate
compound.
3.
1.
h ve low laying d and s-orbitals.
2. the ligand should have at least a donor
the chromate compound is tetrahedral
in shape with delocalised bonds.
atom
3. the central metal ion should be smali
and highly charged.
86. Which of the ligand(s) below islara strong
field splitting ligand(s)?
1. lodo
2. hydroxo
3. carbonyl
94. Carbon in cast iron
lowers its melting
2.
1.
increases Its hardness and decreases
its ductility
3. decreases its hardness and increases
its ductility
87
Tetraammine dicyano vanadium(11)
95. The systematic name of [Co(en),CLINO s
1. bis ethylenediamine dichloro cobaltin
nitrate
1.
is a neutral complex
2.
can exhibit geometric isomerism
3.
is an octahedral complex
bis ethylene diamine dichloro cobaitale
(i) nitrate
3. dichlorobisethylene diamine coball (i)
nitrate
2.
88. Which of the following ligands can serve in
linkage isomerism
1. C N]
96. Optical isomerism in complexes
1. have mirror image isomers that are
nonsuperimposible
2. have isomers that are physically
identical in all ways.
3. have isomers that differ in the way they
rotate the plane of polarised light.
2.
3.
89. Complex ions formed by d-block elements
may
1.
cationic
2. neutral
3. anionic
90. Across the first series of d-block elements
1.
first ionisation energy gradual
decreases with decrease in atomic
number of elements.
2. electrons are filling theh 4s subshell as
well.
3. atomic radius generally decreases as
eifective nuclear charge increases.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5a6d9c67-6f13-49d2-ac4d-2d996f90a88b%2F588faebb-20aa-442b-8ada-8130e020852d%2F5lxd2lr_processed.jpeg&w=3840&q=75)
Transcribed Image Text:o Scandium and Zinc are not consideren
transition elements because they
1 do not form complexes
2 exist only in one oxidation state
do not form ions in which the 34.
84 The olements in between Sc-Zn
1.
all show paramagnetism
2 form ocloured hydrated ions in aqueous
solution
3.
3. form ions that hive incompletely filled
3d-subshell.
subshell is partially filled.
92 d-biock element
85 This question relates to compounds of
chromium
1.
have variable oxidation state
1.
their +2 oxidation state is common at
2.
the beginning of the series.
3. form complex ions
chromium can form chormate(VII) and
dichromate (Vil) compounds which are
respectively orange and yellow in
colour
2.
In order to form a complex ion
the central metal ion or atom shoul
93.
the dichromate compound is made by
aciditying solutions of the chromate
compound.
3.
1.
h ve low laying d and s-orbitals.
2. the ligand should have at least a donor
the chromate compound is tetrahedral
in shape with delocalised bonds.
atom
3. the central metal ion should be smali
and highly charged.
86. Which of the ligand(s) below islara strong
field splitting ligand(s)?
1. lodo
2. hydroxo
3. carbonyl
94. Carbon in cast iron
lowers its melting
2.
1.
increases Its hardness and decreases
its ductility
3. decreases its hardness and increases
its ductility
87
Tetraammine dicyano vanadium(11)
95. The systematic name of [Co(en),CLINO s
1. bis ethylenediamine dichloro cobaltin
nitrate
1.
is a neutral complex
2.
can exhibit geometric isomerism
3.
is an octahedral complex
bis ethylene diamine dichloro cobaitale
(i) nitrate
3. dichlorobisethylene diamine coball (i)
nitrate
2.
88. Which of the following ligands can serve in
linkage isomerism
1. C N]
96. Optical isomerism in complexes
1. have mirror image isomers that are
nonsuperimposible
2. have isomers that are physically
identical in all ways.
3. have isomers that differ in the way they
rotate the plane of polarised light.
2.
3.
89. Complex ions formed by d-block elements
may
1.
cationic
2. neutral
3. anionic
90. Across the first series of d-block elements
1.
first ionisation energy gradual
decreases with decrease in atomic
number of elements.
2. electrons are filling theh 4s subshell as
well.
3. atomic radius generally decreases as
eifective nuclear charge increases.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning