9. A binary ionic compound crystallizes in the cubic unit cell shown below. The anions (larger ST ol spheres) define the unit cell while four cations (smaller dark spheres) are located in tetrahedral holes within the unit cell. What is the formula for this ionic compound (M = cation, X= anion)? bifor biloe 200 b io a. MX b. M2X MX2 d. M2X3 с. e. MX4
9. A binary ionic compound crystallizes in the cubic unit cell shown below. The anions (larger ST ol spheres) define the unit cell while four cations (smaller dark spheres) are located in tetrahedral holes within the unit cell. What is the formula for this ionic compound (M = cation, X= anion)? bifor biloe 200 b io a. MX b. M2X MX2 d. M2X3 с. e. MX4
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section: Chapter Questions
Problem 42GQ: Tungsten crystallizes in the unit cell shown here. Unit cell for tungsten (a) What type of unit cell...
Related questions
Question
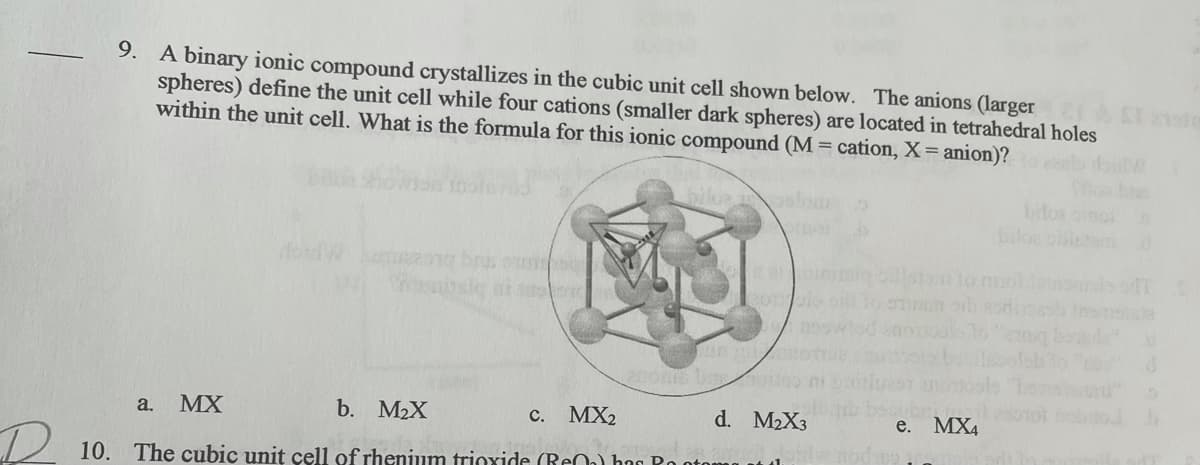
Transcribed Image Text:9. A binary ionic compound crystallizes in the cubic unit cell shown below. The anions (larger
spheres) define the unit cell while four cations (smaller dark spheres) are located in tetrahedral holes
within the unit cell. What is the formula for this ionic compound (M = cation, X= anion)?
eelo bi
biloa
bifoa oino
biloe ci
le of
hsk at s
sta
P.
MX
b. M2X
MX2
d. M2X3
e. MX4
a.
с.
D.
10. The cubic uniț çell of rhenium trioxide (ReOa) hoo Po
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning