
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 3PS
A portion of the crystalline lattice for potassium is illustrated below.
- (a) In what type of unit cell are the K atoms arranged?
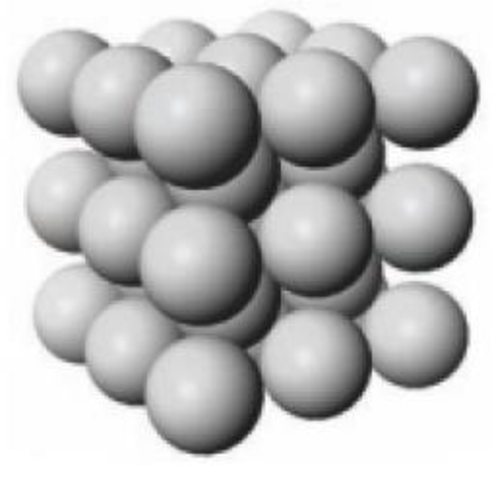
A portion of the solid-state structure of potassium.
- (b) If one edge of the potassium unit cell is 533 pm, what is the density of potassium?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
If the density of europium is 5.25 g/cm3 at 20 degrees celsius, what is the mass (in grams)of one unit cell?
An element has a crystal structure in which the width of the cubic unit cell equals the diameter of an atom. What type of unit cell does it have?
What is the formula of a solid containing A,B, and C atoms in a cubic lattice in which the A atoms occupy the corners, the B atoms the body-center position, and the C atoms the faces of the unit cell?
What is the formula for this?
Chapter 12 Solutions
Chemistry & Chemical Reactivity
Ch. 12.1 - (a) Determining an Atom Radius from Lattice...Ch. 12.2 - If an ionic solid has an fcc lattice of anions (X)...Ch. 12.2 - Potassium chloride has the same unit cell as NaCl....Ch. 12.6 - Prob. 1.1ACPCh. 12.6 - Describe the unit cell of lithium (see Figure).Ch. 12.6 - Prob. 1.3ACPCh. 12.6 - Prob. 1.4ACPCh. 12.6 - Prob. 2.1ACPCh. 12.6 - Prob. 2.2ACPCh. 12.6 - Prob. 2.3ACP
Ch. 12.6 - How many tin atoms are contained in the tetragonal...Ch. 12.6 - Prob. 3.2ACPCh. 12.6 - Prob. 3.3ACPCh. 12.6 - Prob. 3.4ACPCh. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - A portion of the crystalline lattice for potassium...Ch. 12 - The unit cell of silicon carbide, SiC, is...Ch. 12 - Prob. 5PSCh. 12 - Rutile, TiO2, crystallizes in a structure...Ch. 12 - Cuprite is a semiconductor. Oxide ions are at the...Ch. 12 - The mineral fluorite, which is composed of calcium...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - The density of copper metal is 8.95 g/cm3. If the...Ch. 12 - Potassium iodide has a face-centered cubic unit...Ch. 12 - A unit cell of cesium chloride is illustrated in...Ch. 12 - Predict the trend in lattice energy, from least...Ch. 12 - Prob. 14PSCh. 12 - To melt an ionic solid, energy must be supplied to...Ch. 12 - Which compound in each of the following pairs...Ch. 12 - Prob. 17PSCh. 12 - Prob. 18PSCh. 12 - Considering only the molecular orbitals formed by...Ch. 12 - Prob. 20PSCh. 12 - Prob. 21PSCh. 12 - Prob. 22PSCh. 12 - Prob. 23PSCh. 12 - Prob. 24PSCh. 12 - Prob. 25PSCh. 12 - Prob. 26PSCh. 12 - Prob. 27PSCh. 12 - Prob. 28PSCh. 12 - A diamond unit cell is shown here. Unit cell of...Ch. 12 - The structure of graphite is given in Figure...Ch. 12 - We have identified six types of solids (metallic,...Ch. 12 - Prob. 32PSCh. 12 - Classify each of the following materials as...Ch. 12 - Prob. 34PSCh. 12 - Benzene, C6H6, is an organic liquid that freezes...Ch. 12 - The specific heat capacity of silver is 0.235 J/g ...Ch. 12 - Prob. 37PSCh. 12 - Prob. 38PSCh. 12 - Prob. 39PSCh. 12 - If your air conditioner is more than several years...Ch. 12 - Sketch a phase diagram for O2 from the following...Ch. 12 - Tungsten crystallizes in the unit cell shown here....Ch. 12 - Silver crystallizes in a face-centered cubic unit...Ch. 12 - The unit cell shown here is for calcium carbide....Ch. 12 - The very dense metal iridium has a face-centered...Ch. 12 - Vanadium metal has a density of 6.11 g/cm3....Ch. 12 - Prob. 47GQCh. 12 - Prob. 48GQCh. 12 - Prob. 49GQCh. 12 - Consider the three types of cubic units cells. (a)...Ch. 12 - The solid-state structure of silicon is shown...Ch. 12 - The solid-state structure of silicon carbide is...Ch. 12 - Spinels are solids with the general formula AB2O4...Ch. 12 - Using the thermochemical data below and an...Ch. 12 - Prob. 55GQCh. 12 - Prob. 56GQCh. 12 - Prob. 57GQCh. 12 - Prob. 58GQCh. 12 - Prob. 59GQCh. 12 - Prob. 60GQCh. 12 - Like ZnS, lead(II) sulfide, PbS (commonly called...Ch. 12 - CaTiO3, a perovskite, has the structure below. (a)...Ch. 12 - Potassium bromide has the same lattice structure...Ch. 12 - Calculate the lattice energy of CaCl2 using a...Ch. 12 - Why is it not possible for a salt with the formula...Ch. 12 - Prob. 67SCQCh. 12 - Prob. 68SCQCh. 12 - Prob. 69SCQCh. 12 - Phase diagrams for materials that have allotropes...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rutile, TiO2, crystallizes in a structure characteristic of many other ionic compounds How many formula units of TiO2 are in the unit cell illustrated here? (The oxide ions marked by an x are wholly within the cell; the others are in the cell faces.) Unit cell for rufflearrow_forwardThe unit cell of silicon carbide, SiC, is illustrated below. (a) In what type of unit cell are the (dark gray) C atoms arranged? (b) If one edge of the silicon carbide unit cell is 436.0 pm, what is the calculated density of this compound? A portion of the solid-state structure of silicon carbide.arrow_forwardWhat is the coordination number of Cs in CsCl? of Na in NaCl? of Zn2 in ZnS?arrow_forward
- Describe the characteristic features of a simple cubic unit cell?arrow_forwardAssume that the diameter of a metal atom is 5 nm. What is the total volume (in nm3) occupied by a face-centered cubic unit cell?arrow_forwardThe metal cesium crystallizes in a body-centered cubic lattice. If the density of cesium is 1.88 g/cm3, what is the unit cell volume?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY