9. Consider the chemical reaction below: 5x C-C | C-C + CC What will happen to AG if the reaction temperature is decreased? (a) AG becomes more negative, making the reaction spontaneous. (b) AG becomes more positive, making the reaction spontaneous. (c) AG becomes more negative, making the reaction non-spontaneous. (d) AG becomes more positive, making the reaction non-spontaneous. (e) AG is unaffected by changes in temperature.
9. Consider the chemical reaction below: 5x C-C | C-C + CC What will happen to AG if the reaction temperature is decreased? (a) AG becomes more negative, making the reaction spontaneous. (b) AG becomes more positive, making the reaction spontaneous. (c) AG becomes more negative, making the reaction non-spontaneous. (d) AG becomes more positive, making the reaction non-spontaneous. (e) AG is unaffected by changes in temperature.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter16: Thermodynamics
Section: Chapter Questions
Problem 37E: Consider the decomposition of red mercury(II) oxide under standard state conditions.....
Related questions
Question
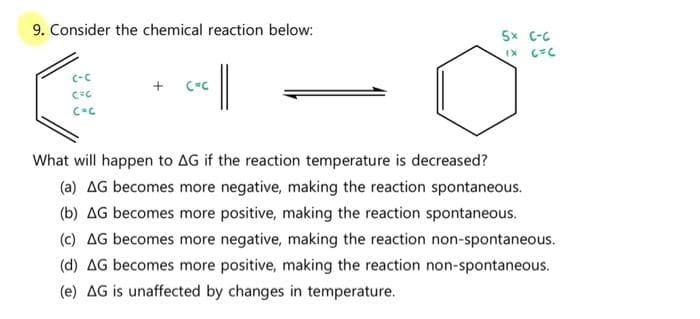
Transcribed Image Text:9. Consider the chemical reaction below:
5x C-C
C-C
What will happen to AG if the reaction temperature is decreased?
(a) AG becomes more negative, making the reaction spontaneous.
(b) AG becomes more positive, making the reaction spontaneous.
(c) AG becomes more negative, making the reaction non-spontaneous.
(d) AG becomes more positive, making the reaction non-spontaneous.
(e) AG is unaffected by changes in temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning