For the decomposition of solid calcium carbonate at 25 C, CaCO3(s) CaO(s) + CO2(g) (a) Calculate rG using values of fG. Is the reaction product-favored at equilibrium? (b) Calculate rG when the partial pressure of carbon dioxide is 0.10 atm in the presence of both calcium carbonate and calcium oxide. Is the reaction spontaneous under these conditions?
For the decomposition of solid calcium carbonate at 25 C, CaCO3(s) CaO(s) + CO2(g) (a) Calculate rG using values of fG. Is the reaction product-favored at equilibrium? (b) Calculate rG when the partial pressure of carbon dioxide is 0.10 atm in the presence of both calcium carbonate and calcium oxide. Is the reaction spontaneous under these conditions?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section: Chapter Questions
Problem 32PS
Related questions
Question
Aren’t caco3 and CaO, why do they have partial pressure
Transcribed Image Text:4:25
l 4G
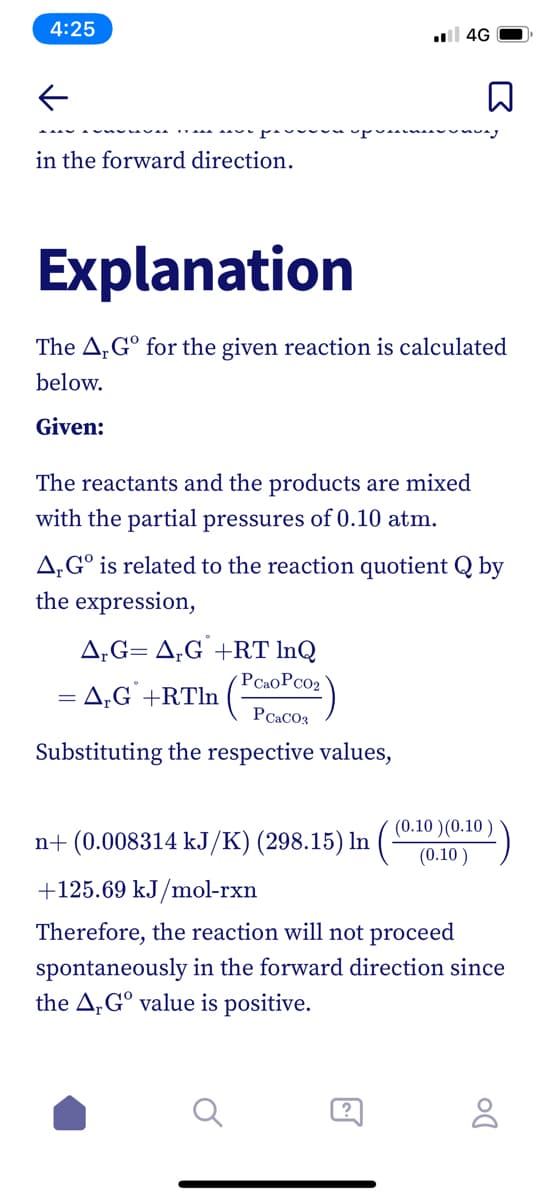
in the forward direction.
Explanation
The A,G° for the given reaction is calculated
below.
Given:
The reactants and the products are mixed
with the partial pressures of 0.10 atm.
A,G° is related to the reaction quotient Q by
the expression,
A,G= A,G +RT InQ
PCaoPcO2
= A;G` +RTln (-
PCACO3
Substituting the respective values,
(0.10 )(0.10
n+ (0.008314 kJ/K) (298.15) In
(0.10 )
+125.69 kJ/mol-rxn
Therefore, the reaction will not proceed
spontaneously in the forward direction since
the A,G° value is positive.

Transcribed Image Text:4:25
l 4G
Textbook
problem
Principles Of Chemical
Reactivity: Entropy And Free
Energy
For the decomposition of solid
calcium carbonate at 25 C,
CaCO3(s) CaO(s) + CO2(g) (a)
Calculate rG using values of fG.
Is the reaction product-favored at
equilibrium? (b) Calculate rG
when the partial pressure of
carbon dioxide is 0.10 atm in the
presence of both calcium
carbonate and calcium oxide. Is
the reaction spontaneous under
these conditions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
