9. Consider the following compound: FeCl, a. What is the name of the compound? ( b. What is the percent by mass of chlorine in FeCl,? Show your work. c. FeCl, decomposes and produces 2.12 g of chlorine gas. What mass of FeCl, decomposed? Show your work. ( no of oblorine are contained in
9. Consider the following compound: FeCl, a. What is the name of the compound? ( b. What is the percent by mass of chlorine in FeCl,? Show your work. c. FeCl, decomposes and produces 2.12 g of chlorine gas. What mass of FeCl, decomposed? Show your work. ( no of oblorine are contained in
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter4: Determination Of A Chemical Formula
Section: Chapter Questions
Problem 2ASA: If one can find the ratio of the number of moles of the elements in a compound to one another, one...
Related questions
Question
can you solve these questions with work shown
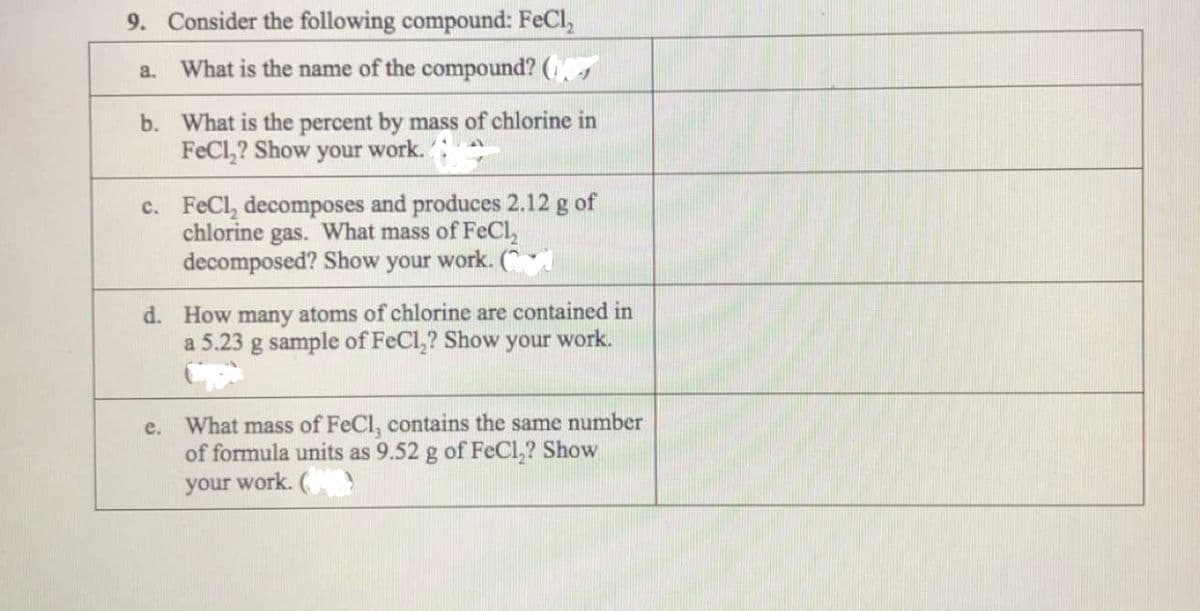
Transcribed Image Text:9. Consider the following compound: FeCl,
What is the name of the compound? (s
a.
b. What is the percent by mass of chlorine in
FeCl,? Show your work.:
c. FeCl, decomposes and produces 2.12 g of
chlorine gas. What mass of FeCl,
decomposed? Show your work. (
d. How many atoms of chlorine are contained in
a 5.23 g sample of FeCl,? Show your work.
What mass of FeCl, contains the same number
of formula units as 9.52 g of FeCl,? Show
your work. (
е.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning