900 800 Carbon disulfide 700 600 Methanol 500 Ethanol 400 Heptane 300 200 100 0. 10 20 30 40 50 60 70 80 90 100 110 Temperature (°C) The vapor pressure of diethyl ether is 400 mm Hg at 18.0 °C. From the plot of vapor pressures vs temperature above, estimate the temperature at which the vapor pressure of heptane is 400 mm Hg. °C The heat of vaporization of diethyl ether would be expected to be O than the heat of vaporization of heptane. Vapor pressure (mm Hg)
900 800 Carbon disulfide 700 600 Methanol 500 Ethanol 400 Heptane 300 200 100 0. 10 20 30 40 50 60 70 80 90 100 110 Temperature (°C) The vapor pressure of diethyl ether is 400 mm Hg at 18.0 °C. From the plot of vapor pressures vs temperature above, estimate the temperature at which the vapor pressure of heptane is 400 mm Hg. °C The heat of vaporization of diethyl ether would be expected to be O than the heat of vaporization of heptane. Vapor pressure (mm Hg)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 19PS
Related questions
Question
100%
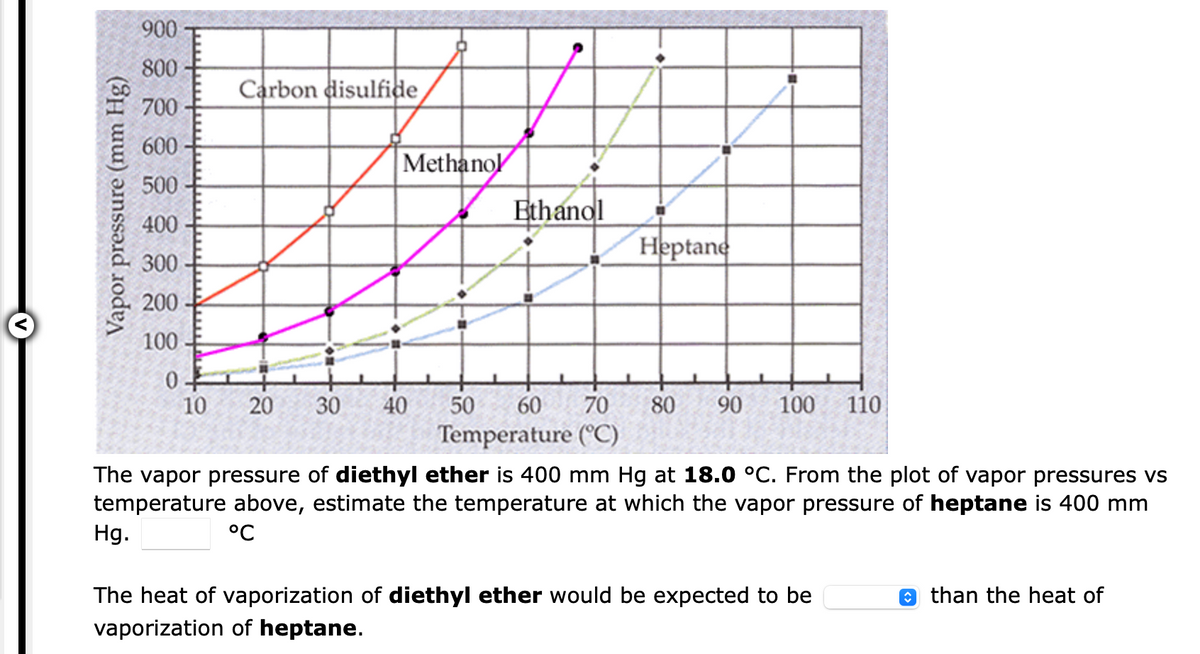
Transcribed Image Text:900
800
Carbon disulfide
700
600
Methanol
500
Ethanol
400
Heptane
300
200
100
0.
10
20
30
40
50
60 70
80
90
100
110
Temperature (°C)
The vapor pressure of diethyl ether is 400 mm Hg at 18.0 °C. From the plot of vapor pressures vs
temperature above, estimate the temperature at which the vapor pressure of heptane is 400 mm
Hg.
°C
The heat of vaporization of diethyl ether would be expected to be
e than the heat of
vaporization of heptane.
Vapor pressure (mm Hg)
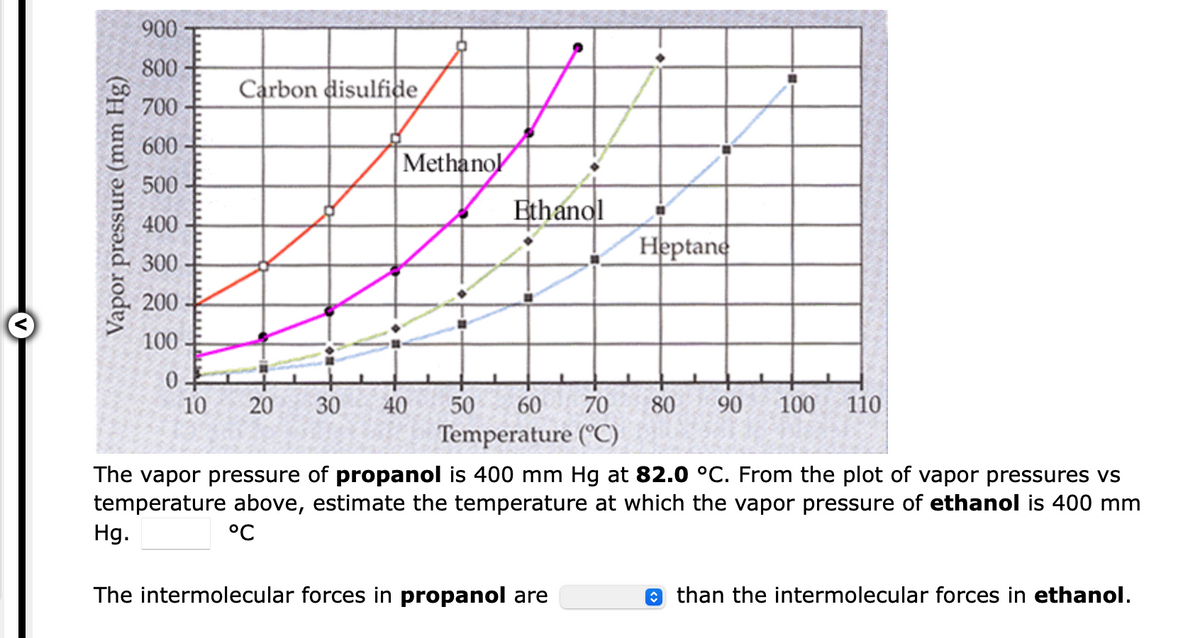
Transcribed Image Text:900
800
Carbon disulfide
700
600
Methanol
500
Ethanol
400
Heptane
300
200
100
0.
10
20
30
40
50
60 70
80
90
100
110
Temperature (°C)
The vapor pressure of propanol is 400 mm Hg at 82.0 °C. From the plot of vapor pressures vs
temperature above, estimate the temperature at which the vapor pressure of ethanol is 400 mm
Hg.
°C
The intermolecular forces in propanol are
o than the intermolecular forces in ethanol.
Vapor pressure (mm Hg)
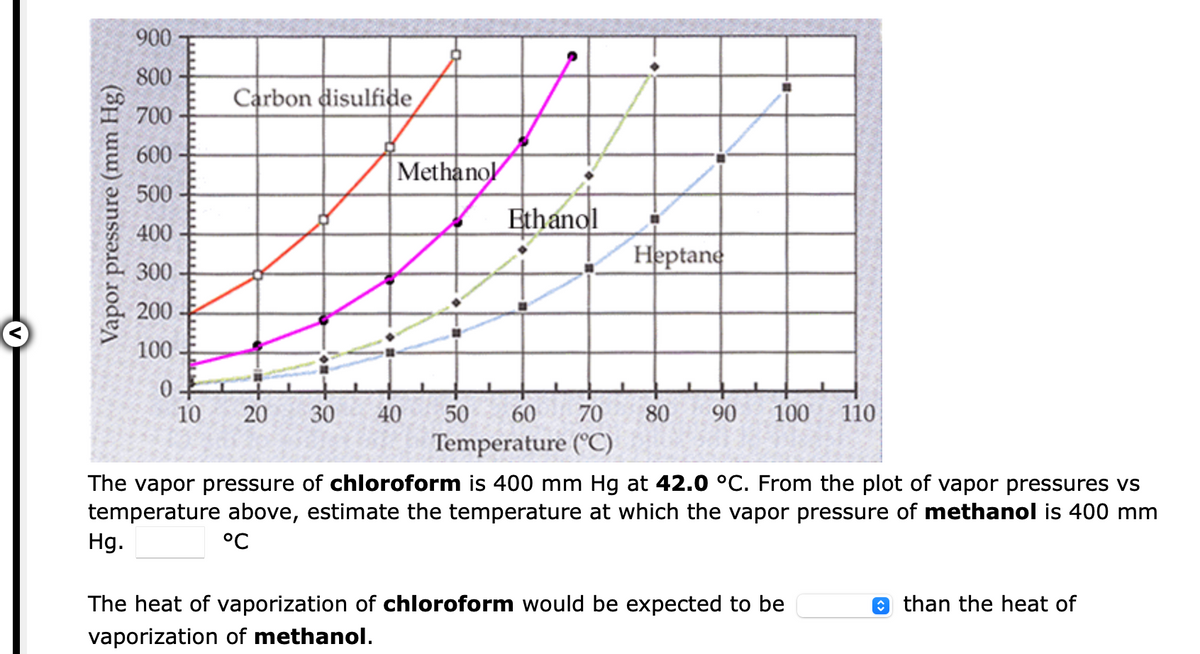
Transcribed Image Text:900
800
Carbon disulfide
700
600
Methanol
500
Ethanol
400
Heptane
300
200
100
10
30
40
50
60
70
80
90
100
110
Temperature (°C)
The vapor pressure of chloroform is 400 mm Hg at 42.0 °C. From the plot of vapor pressures vs
temperature above, estimate the temperature at which the vapor pressure of methanol is 400 mm
Hg.
°C
The heat of vaporization of chloroform would be expected to be
O than the heat of
vaporization of methanol.
Vapor pressure (mm Hg)
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT