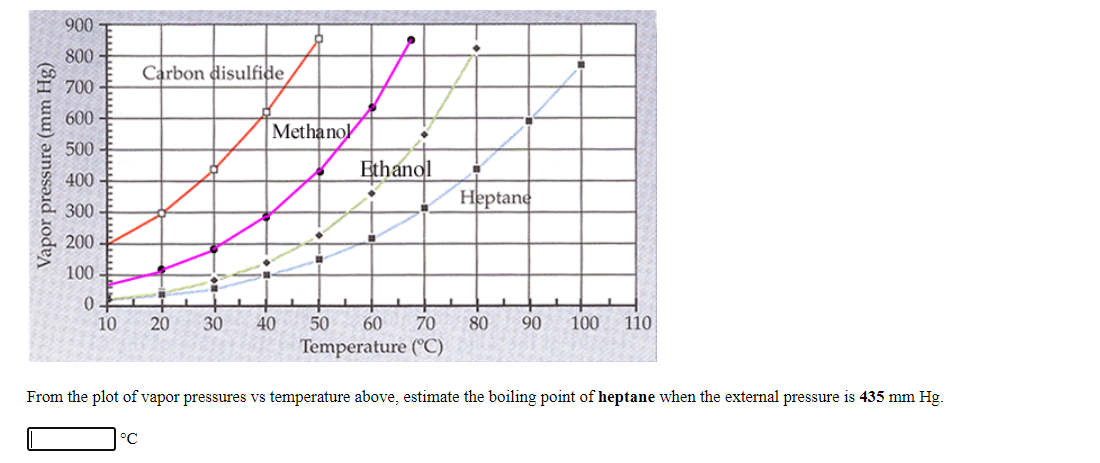
900 800 Carbon disulfide 700 600 Methanol 500 Ethanol 400 Heptane 300 200 100 10 20 30 40 50 60 70 80 90 100 110 Temperature (°C) From the plot of vapor pressures vs temperature above, estimate the boiling point of heptane when the external pressure is 435 mm Hg. °C Vapor pressure (mm Hg)
900 800 Carbon disulfide 700 600 Methanol 500 Ethanol 400 Heptane 300 200 100 10 20 30 40 50 60 70 80 90 100 110 Temperature (°C) From the plot of vapor pressures vs temperature above, estimate the boiling point of heptane when the external pressure is 435 mm Hg. °C Vapor pressure (mm Hg)
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter6: Steam Distillation, Vacuum Distillation, And Sublimation
Section: Chapter Questions
Problem 6Q: A mixture of toluene (bp110.8C) and water is steam distilled. Visual inspection of the distillate...
Related questions
Question
Transcribed Image Text:900
800
Carbon disulfide
700
600
Methanol
500
Ethanol
400
Heptane
300
200
100
10
20
30
40
50
60
70
80
90
100
110
Temperature (°C)
From the plot of vapor pressures vs temperature above, estimate the boiling point of heptane when the external pressure is 435 mm Hg.
°C
Vapor pressure (mm Hg)
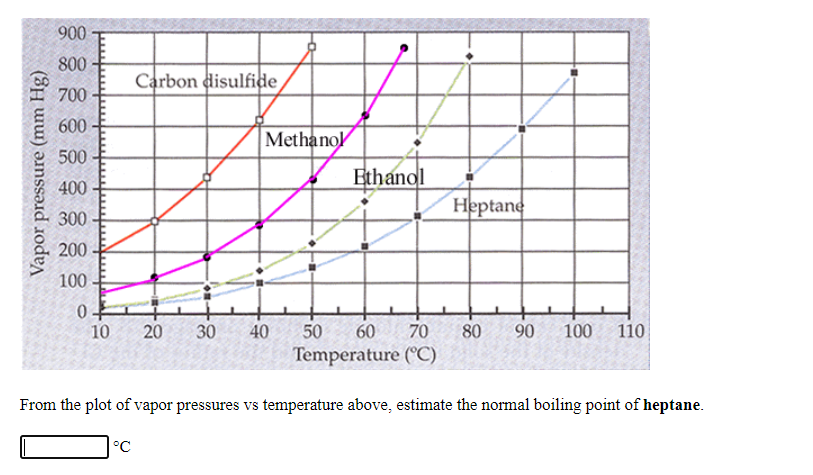
Transcribed Image Text:900
800
Carbon disulfide
700
600
Methanol
500
Ethanol
400
Heptane
300
200
100
10
20
30
40
50
60
70
80
90
100
110
Temperature (°C)
From the plot of vapor pressures vs temperature above, estimate the normal boiling point of heptane.
°C
Vapor pressure (mm Hg)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT