9:55 PM 76 B/s Edit CHEM 202 ASSIGNMENT # 1 – DUE ON WEDNESDAY, 8TH APRIL 2020 1. Give the IUPAC name or draw the structure, whichever is required for each of the following: осHз COOH OH (a) (b) (c) -CH3 NH2 CI (d) 4-hydroxybenzaldehyde Bromobenzyl chloride (h) 2,4-dimethylaniline 2. Use the following list of compounds to answer the questions below: [4 pts. each] (e) p-methoxytoluene (f) p-Nitroanisole (bg) m- D Н НС AB AC AD AE BC Select the compound that is best described as: (a) A neutral antiaromatic aromatic system electron system (d) A non-aromatic, conjugated, 6 pi (b) An aromatic system with n= 2 in the 4n+2 Huckel rule (e) A 6 pi electron aromatic system (c) A neutral, 4 p-electron, anti-aromatic system 3. Identify the molecule/species which are aromatic and those which are anti-aromatic: 4. What is the formula of the electrophilic reagent/species present in the following reaction? 88 Tools Mobile View Share 9:56 PM Edit Br, ? FeBr3 A single organic product was isolated after Birch reduction of p-xylene. Suggest a reasonable structure for this substance. 5. 6. Chromic oxidation of 4-t-butyl-1,2-dimethylbenzene yielded a single compound having the molecular formula C12H1404. What was this compound? 7. Nitration of 1,4-dimethylbenzene (p-xylene) gives a single product with mol. formula CsH9NO2 in high yield. What is the product? 8. What product would you expect from the bromination of p-methylbenzoic acid? 9. From the following list, identify the groups which are activating and ortho, para directors and those which are deactivating, meta directors when attached to a benzene ring: -ci ; -N(CH3)2 ; -NO2 ; -ОСНз ; -NHCOCH3. 10. Give the structure of the principal organic product formed on reaction of benzyl bromide with each of the following reagents: (a) sodium ethoxide (b) potassium t-butoxide (c) sodium azide (d) sodium hydrogen sulfide (e) sodium iodide (in acetone) 11. Order the following compounds according to their activating substituents (from least activating to most activating: NO2 NH. осн, ÇOOH (1) (11) (iv) (v) 12. Rank the following in terms of increasing reactivity toward nitration with HNO3, H2SO4 (least to most): NHCH, 13. Rank the following in each group in order of their reactivity toward electrophilic substitution: (a) Nitrobenzene, phenol, toluene, benzene (b) Phenol, benzene, chlorobenzene, benzoic acid (c) Benzene, bromobenzene, benzaldehyde, aniline 14. Give the major product(s) for each of the following reactions. Indicate whether the reaction proceeds faster or slower than the corresponding reaction of benzene. NO2 HNO3 so, (a) (b) H2S0 4 H2SO 4 В г, (c) FeB r3 15. Write the formula of the electrophilic reagent/species present in each reaction of the preceding problem (#9). 16. Provide the reactant, reagent, or product omitted from each of the following: 88 DO Tools Mobile View Share
9:55 PM 76 B/s Edit CHEM 202 ASSIGNMENT # 1 – DUE ON WEDNESDAY, 8TH APRIL 2020 1. Give the IUPAC name or draw the structure, whichever is required for each of the following: осHз COOH OH (a) (b) (c) -CH3 NH2 CI (d) 4-hydroxybenzaldehyde Bromobenzyl chloride (h) 2,4-dimethylaniline 2. Use the following list of compounds to answer the questions below: [4 pts. each] (e) p-methoxytoluene (f) p-Nitroanisole (bg) m- D Н НС AB AC AD AE BC Select the compound that is best described as: (a) A neutral antiaromatic aromatic system electron system (d) A non-aromatic, conjugated, 6 pi (b) An aromatic system with n= 2 in the 4n+2 Huckel rule (e) A 6 pi electron aromatic system (c) A neutral, 4 p-electron, anti-aromatic system 3. Identify the molecule/species which are aromatic and those which are anti-aromatic: 4. What is the formula of the electrophilic reagent/species present in the following reaction? 88 Tools Mobile View Share 9:56 PM Edit Br, ? FeBr3 A single organic product was isolated after Birch reduction of p-xylene. Suggest a reasonable structure for this substance. 5. 6. Chromic oxidation of 4-t-butyl-1,2-dimethylbenzene yielded a single compound having the molecular formula C12H1404. What was this compound? 7. Nitration of 1,4-dimethylbenzene (p-xylene) gives a single product with mol. formula CsH9NO2 in high yield. What is the product? 8. What product would you expect from the bromination of p-methylbenzoic acid? 9. From the following list, identify the groups which are activating and ortho, para directors and those which are deactivating, meta directors when attached to a benzene ring: -ci ; -N(CH3)2 ; -NO2 ; -ОСНз ; -NHCOCH3. 10. Give the structure of the principal organic product formed on reaction of benzyl bromide with each of the following reagents: (a) sodium ethoxide (b) potassium t-butoxide (c) sodium azide (d) sodium hydrogen sulfide (e) sodium iodide (in acetone) 11. Order the following compounds according to their activating substituents (from least activating to most activating: NO2 NH. осн, ÇOOH (1) (11) (iv) (v) 12. Rank the following in terms of increasing reactivity toward nitration with HNO3, H2SO4 (least to most): NHCH, 13. Rank the following in each group in order of their reactivity toward electrophilic substitution: (a) Nitrobenzene, phenol, toluene, benzene (b) Phenol, benzene, chlorobenzene, benzoic acid (c) Benzene, bromobenzene, benzaldehyde, aniline 14. Give the major product(s) for each of the following reactions. Indicate whether the reaction proceeds faster or slower than the corresponding reaction of benzene. NO2 HNO3 so, (a) (b) H2S0 4 H2SO 4 В г, (c) FeB r3 15. Write the formula of the electrophilic reagent/species present in each reaction of the preceding problem (#9). 16. Provide the reactant, reagent, or product omitted from each of the following: 88 DO Tools Mobile View Share
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.28P
Related questions
Question
100%
Nitration of 1,4-dimethyl beneze
![9:55 PM
76
B/s
Edit
CHEM 202 ASSIGNMENT # 1 – DUE ON WEDNESDAY, 8TH APRIL 2020
1. Give the IUPAC name or draw the structure, whichever is required for each of the following:
осHз
COOH
OH
(a)
(b)
(c)
-CH3
NH2
CI
(d) 4-hydroxybenzaldehyde
Bromobenzyl chloride (h) 2,4-dimethylaniline
2. Use the following list of compounds to answer the questions below: [4 pts. each]
(e) p-methoxytoluene
(f) p-Nitroanisole (bg) m-
D
Н
НС
AB
AC
AD
AE
BC
Select the compound that is best described as:
(a) A neutral antiaromatic aromatic system
electron system
(d) A non-aromatic, conjugated, 6 pi
(b) An aromatic system with n= 2 in the 4n+2 Huckel rule (e) A 6 pi electron aromatic
system
(c) A neutral, 4 p-electron, anti-aromatic system
3. Identify the molecule/species which are aromatic and those which are anti-aromatic:
4. What is the formula of the electrophilic reagent/species present in the following reaction?
88
Tools
Mobile View
Share](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F82621bbd-9e3d-4b8c-b83e-254d0a849f1a%2F0592bb5f-6815-4842-9dbb-314422fa71da%2F4jony1h.png&w=3840&q=75)
Transcribed Image Text:9:55 PM
76
B/s
Edit
CHEM 202 ASSIGNMENT # 1 – DUE ON WEDNESDAY, 8TH APRIL 2020
1. Give the IUPAC name or draw the structure, whichever is required for each of the following:
осHз
COOH
OH
(a)
(b)
(c)
-CH3
NH2
CI
(d) 4-hydroxybenzaldehyde
Bromobenzyl chloride (h) 2,4-dimethylaniline
2. Use the following list of compounds to answer the questions below: [4 pts. each]
(e) p-methoxytoluene
(f) p-Nitroanisole (bg) m-
D
Н
НС
AB
AC
AD
AE
BC
Select the compound that is best described as:
(a) A neutral antiaromatic aromatic system
electron system
(d) A non-aromatic, conjugated, 6 pi
(b) An aromatic system with n= 2 in the 4n+2 Huckel rule (e) A 6 pi electron aromatic
system
(c) A neutral, 4 p-electron, anti-aromatic system
3. Identify the molecule/species which are aromatic and those which are anti-aromatic:
4. What is the formula of the electrophilic reagent/species present in the following reaction?
88
Tools
Mobile View
Share
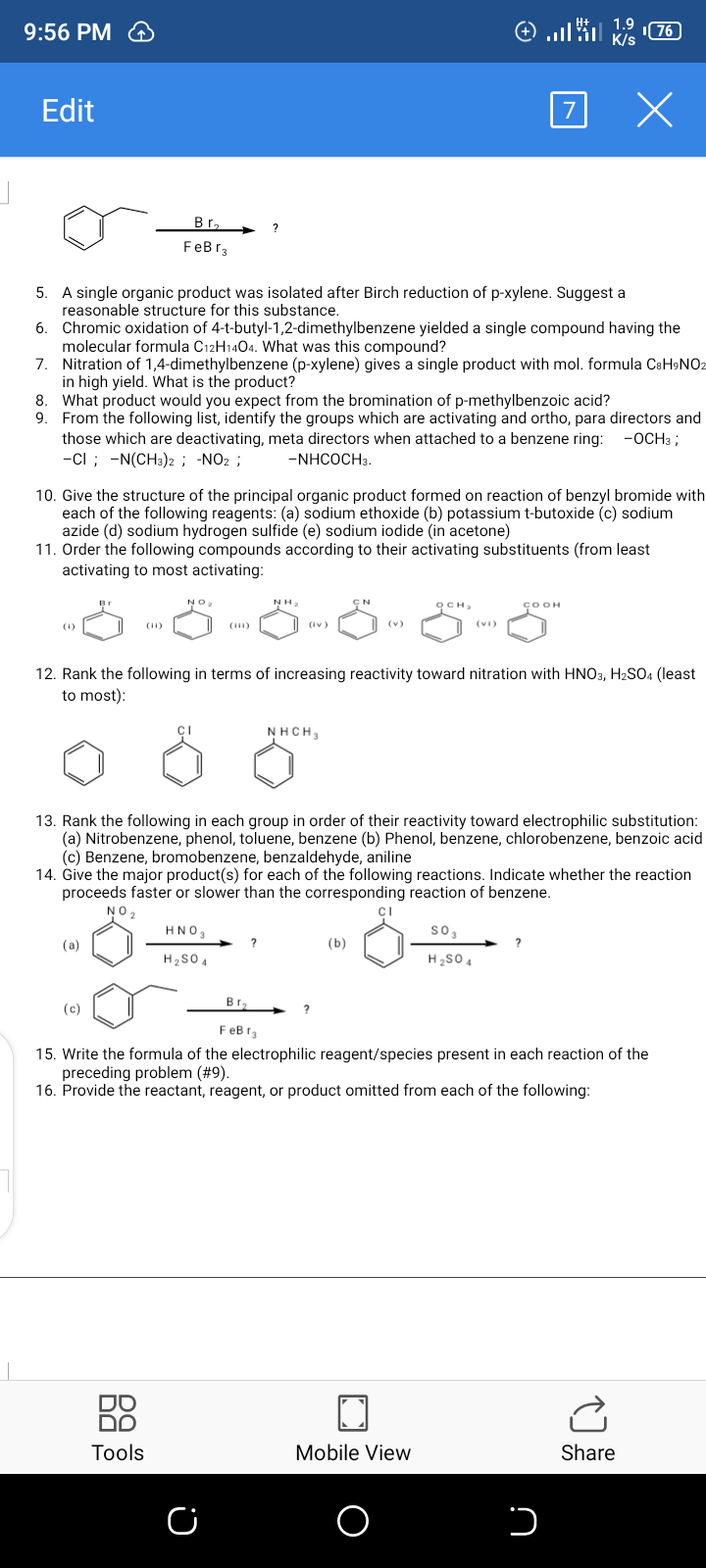
Transcribed Image Text:9:56 PM
Edit
Br, ?
FeBr3
A single organic product was isolated after Birch reduction of p-xylene. Suggest a
reasonable structure for this substance.
5.
6. Chromic oxidation of 4-t-butyl-1,2-dimethylbenzene yielded a single compound having the
molecular formula C12H1404. What was this compound?
7. Nitration of 1,4-dimethylbenzene (p-xylene) gives a single product with mol. formula CsH9NO2
in high yield. What is the product?
8. What product would you expect from the bromination of p-methylbenzoic acid?
9. From the following list, identify the groups which are activating and ortho, para directors and
those which are deactivating, meta directors when attached to a benzene ring:
-ci ; -N(CH3)2 ; -NO2 ;
-ОСНз ;
-NHCOCH3.
10. Give the structure of the principal organic product formed on reaction of benzyl bromide with
each of the following reagents: (a) sodium ethoxide (b) potassium t-butoxide (c) sodium
azide (d) sodium hydrogen sulfide (e) sodium iodide (in acetone)
11. Order the following compounds according to their activating substituents (from least
activating to most activating:
NO2
NH.
осн,
ÇOOH
(1)
(11)
(iv)
(v)
12. Rank the following in terms of increasing reactivity toward nitration with HNO3, H2SO4 (least
to most):
NHCH,
13. Rank the following in each group in order of their reactivity toward electrophilic substitution:
(a) Nitrobenzene, phenol, toluene, benzene (b) Phenol, benzene, chlorobenzene, benzoic acid
(c) Benzene, bromobenzene, benzaldehyde, aniline
14. Give the major product(s) for each of the following reactions. Indicate whether the reaction
proceeds faster or slower than the corresponding reaction of benzene.
NO2
HNO3
so,
(a)
(b)
H2S0 4
H2SO 4
В г,
(c)
FeB r3
15. Write the formula of the electrophilic reagent/species present in each reaction of the
preceding problem (#9).
16. Provide the reactant, reagent, or product omitted from each of the following:
88
DO
Tools
Mobile View
Share
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,