A 0.32 g soil sample was digested and diluted to 481-mL. The diluted sample was analyzed for Zn by AAS and found to contain 4.8 mg/L. What is the amount of Zn in the original soil sample in mg/Kg? a. 7215 b. 72150 C. 3608 d. 2405
A 0.32 g soil sample was digested and diluted to 481-mL. The diluted sample was analyzed for Zn by AAS and found to contain 4.8 mg/L. What is the amount of Zn in the original soil sample in mg/Kg? a. 7215 b. 72150 C. 3608 d. 2405
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.14QAP
Related questions
Question
6
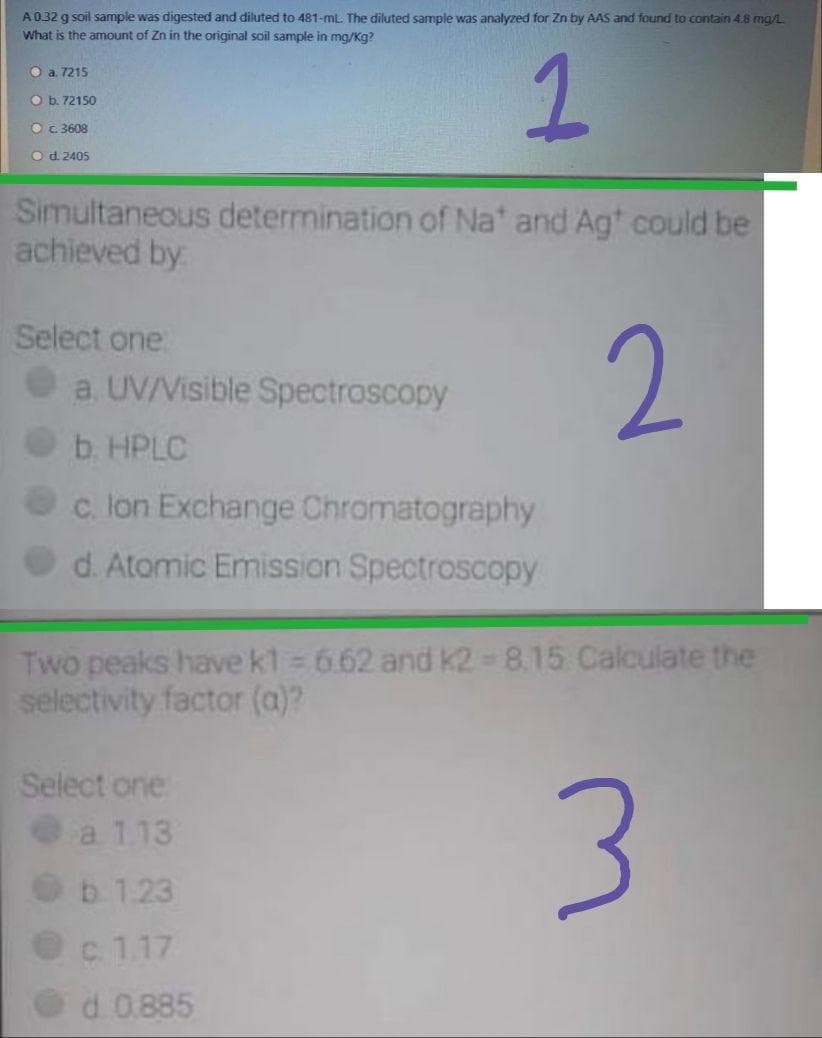
Transcribed Image Text:A032 g soil sample was digested and diluted to 481-ml. The diluted sample was analyzed for Zn by AAS and found to contain 4.8 mg/L
What is the amount of Zn in the original soil sample in mg/Kg?
O a. 7215
O b. 72150
O c3608
Od. 2405
Simultaneous determination of Na' and Agt could be
achieved by
Select one
2.
a. UV/Visible Spectroscopy
b. HPLC
Oc lon Exchange Chromatography
d. Atomic Emission Spectroscopy
Two peaks have kt 6.62 and K2 = 8.15 Calculate the
selectivity factor (a)?
Select one
a 1.13
b. 1.23
c 1.17
d. 0.885
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning