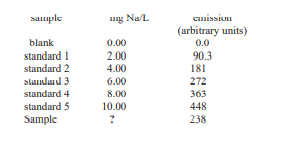
The concentration of Na in plant materials are determined by flame atomic emission. The material to be analyzed is prepared by grinding, homogenizing, and drying at 103 ºC. A sample of approximately 4 g is transferred to a quartz crucible and heated on a hot plate to char the organic material. The sample is heated in a muffle furnace at 550 ºC for several hours. After cooling to room temperature the residue is dissolved by adding 2 mL of 1:1 HNO3 and evaporated to dryness. The residue is redissolved in 10 mL of 1:9 HNO3, filtered and diluted to 50 mL in a volumetric flask. The following data are obtained during a typical analysis for the concentration of Na in a 4.0264-g sample of oat bran. Calculate the weight percent of Na in the plant sample.
The concentration of Na in plant materials are determined by flame atomic emission. The material to be analyzed is prepared by grinding, homogenizing, and drying at 103 ºC. A sample of approximately 4 g is transferred to a quartz crucible and heated on a hot plate to char the organic material. The sample is heated in a muffle furnace at 550 ºC for
several hours. After cooling to room temperature the residue is dissolved by adding 2 mL of 1:1 HNO3 and evaporated to dryness. The residue is redissolved in 10 mL of 1:9 HNO3, filtered and diluted to 50 mL in a volumetric flask. The following data are obtained during a typical analysis for the concentration of Na in a 4.0264-g sample of oat bran. Calculate the weight percent of Na in the plant sample.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images


