A 0.4444 g of organic compound of unknown composition containing carbon, hydrogen and oxygen, is burned with oxygen, and produces 1.124 g of CO2 and 0.1822 g of H20. Calculate the mass of carbon, of hydrogen, and of oxygen that are contained in the organic compound. To obtain the exact answer, use the molar of C being equal to 12.01 g; the molar mass of H 1.010 g; the molar mass of O 16.00 g. In your answer, do not type in the unit gram, just type in the numbers that follow the rules of handling significant figures. Hint 1: Look up the example problem in the next, last question. Hint 2: Consider the law of mass conservation: the mass must be the same before and after a chemical reaction. The mass of carbon is g. The mass of hydrogen is g. The mass of oxygen is g.
A 0.4444 g of organic compound of unknown composition containing carbon, hydrogen and oxygen, is burned with oxygen, and produces 1.124 g of CO2 and 0.1822 g of H20. Calculate the mass of carbon, of hydrogen, and of oxygen that are contained in the organic compound. To obtain the exact answer, use the molar of C being equal to 12.01 g; the molar mass of H 1.010 g; the molar mass of O 16.00 g. In your answer, do not type in the unit gram, just type in the numbers that follow the rules of handling significant figures. Hint 1: Look up the example problem in the next, last question. Hint 2: Consider the law of mass conservation: the mass must be the same before and after a chemical reaction. The mass of carbon is g. The mass of hydrogen is g. The mass of oxygen is g.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter22: Organic And Biological Molecules
Section: Chapter Questions
Problem 157CP
Related questions
Question
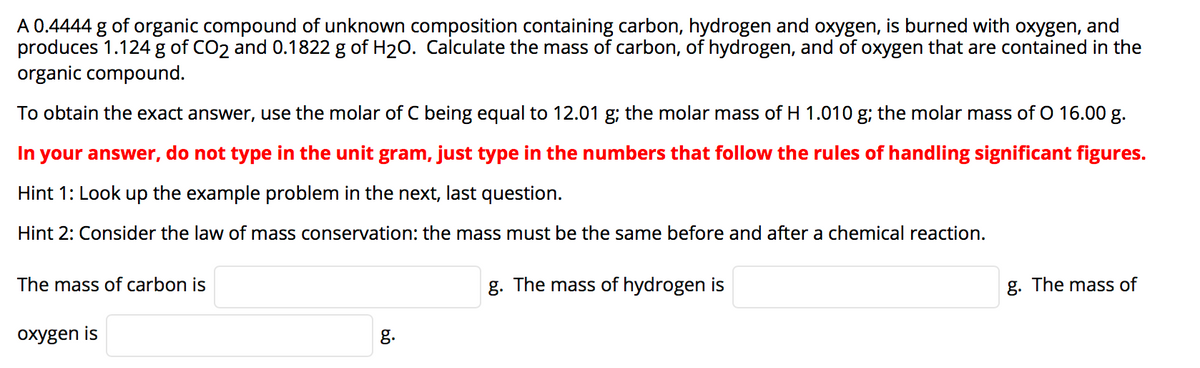
Transcribed Image Text:A 0.4444 g of organic compound of unknown composition containing carbon, hydrogen and oxygen, is burned with oxygen, and
produces 1.124 g of CO2 and 0.1822 g of H20. Calculate the mass of carbon, of hydrogen, and of oxygen that are contained in the
organic compound.
To obtain the exact answer, use the molar of C being equal to 12.01 g; the molar mass of H 1.010 g; the molar mass of O 16.00 g.
In your answer, do not type in the unit gram, just type in the numbers that follow the rules of handling significant figures.
Hint 1: Look up the example problem in the next, last question.
Hint 2: Consider the law of mass conservation: the mass must be the same before and after a chemical reaction.
The mass of carbon is
g. The mass of hydrogen is
g. The mass of
oxygen is
g.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.