With a product weight of 3.77g . Calculate the theoretical yield of product, and use that to calculat the percentage yield of the banana oil product.
With a product weight of 3.77g . Calculate the theoretical yield of product, and use that to calculat the percentage yield of the banana oil product.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterNW2: Nomenclature Worksheet 2: Intro To Naming Functional Groups
Section: Chapter Questions
Problem 21CTQ
Related questions
Question
100%
With a product weight of 3.77g . Calculate the theoretical yield of product, and use that to calculat the percentage yield of the banana oil product.
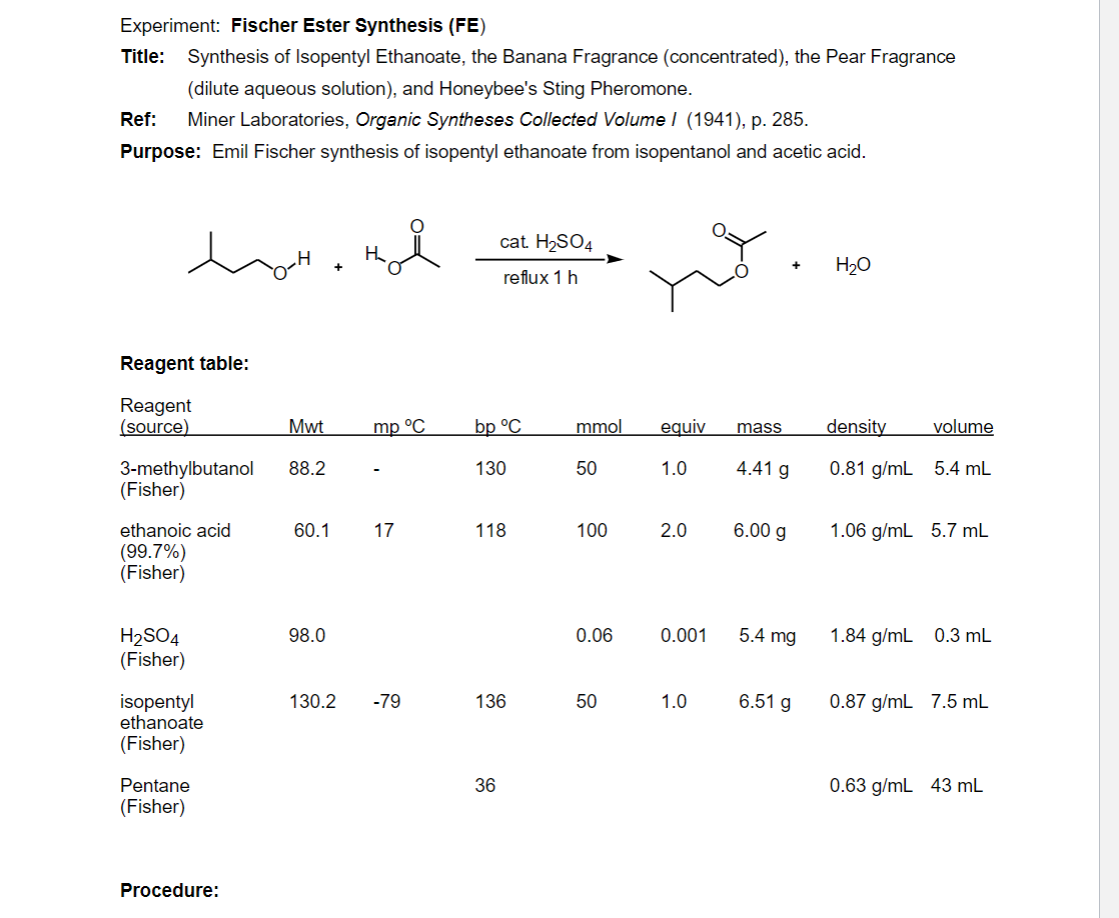
Transcribed Image Text:Experiment: Fischer Ester Synthesis (FE)
Title: Synthesis of Isopentyl Ethanoate, the Banana Fragrance (concentrated), the Pear Fragrance
(dilute aqueous solution), and Honeybee's Sting Pheromone.
Ref:
Miner Laboratories, Organic Syntheses Collected Volume I (1941), p. 285.
Purpose: Emil Fischer synthesis of isopentyl ethanoate from isopentanol and acetic acid.
cat. H2SO4
H2O
reflux 1 h
Reagent table:
Reagent
(source)
Mwt
mp °C
bp °C
mmol
equiv
mass
density
volume
4.41 g
3-methylbutanol
(Fisher)
88.2
130
50
1.0
0.81 g/mL 5.4 mL
ethanoic acid
60.1
17
118
100
2.0
6.00 g
1.06 g/mL 5.7 mL
(99.7%)
(Fisher)
H2SO4
(Fisher)
98.0
0.06
0.001
5.4 mg
1.84 g/mL 0.3 mL
isopentyl
ethanoate
130.2
6.51 g
-79
136
50
1.0
0.87 g/mL 7.5 mL
(Fisher)
Pentane
36
0.63 g/mL 43 mL
(Fisher)
Procedure:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning