A 1-kg block of sub-zero ice is dropped into a large drum of cold water at 0 C. The drum is insulated from outside. Immediately after dropping it, you measured that the ice-block is gaining heat from the water at a rate of 126 W. How much time will it take for all the ice to melt? (no more information is required)
A 1-kg block of sub-zero ice is dropped into a large drum of cold water at 0 C. The drum is insulated from outside. Immediately after dropping it, you measured that the ice-block is gaining heat from the water at a rate of 126 W. How much time will it take for all the ice to melt? (no more information is required)
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 50PE: (a) What is me vapor pressure of water at 20.0C ? (b) What percentage of atmospheric pressure does...
Related questions
Question
A 1-kg block of sub-zero ice is dropped into a large drum of cold water at 0 C. The drum is insulated
from outside. Immediately after dropping it, you measured that the ice-block is gaining heat from
the water at a rate of 126 W. How much time will it take for all the ice to melt? (no more information is required)
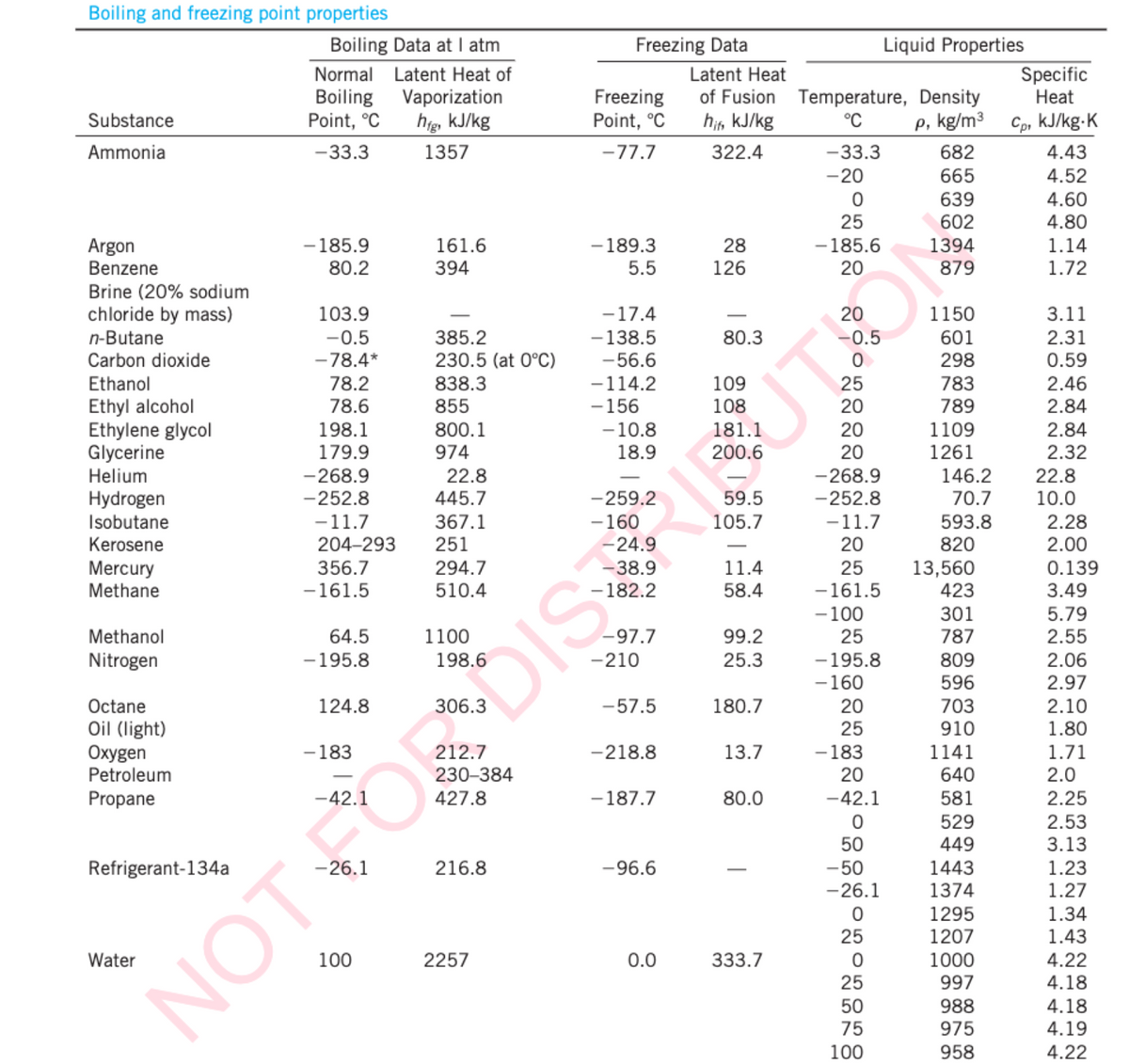
Transcribed Image Text:Boiling and freezing point properties
Substance
Ammonia
Argon
Benzene
Brine (20% sodium
chloride by mass)
n-Butane
Carbon dioxide
Ethanol
Ethyl alcohol
Ethylene glycol
Glycerine
Helium
Hydrogen
Isobutane
Kerosene
Mercury
Methane
Methanol
Nitrogen
Octane
Oil (light)
Oxygen
Petroleum
Propane
Refrigerant-134a
Water
Boiling Data at I atm
Normal Latent Heat of
Boiling
Point, °C
-33.3
-185.9
80.2
103.9
-0.5
-78.4*
78.2
78.6
198.1
179.9
-268.9
-252.8
-11.7
204-293
356.7
-161.5
64.5
-195.8
124.8
-183
100
Vaporization
hig, kJ/kg
1357
161.6
394
385.2
230.5 (at 0°C)
838.3
855
800.1
974
22.8
445.7
367.1
251
294.7
510.4
1100
230-384
427.8
216.8
2257
Freezing Data
Freezing
Point, °C
-77.7
- 189.3
5.5
-17.4
-138.5
-56.6
-114.2
-156
-10.8
18.9
-259.2
- 160
-24.9
NOT FOR DIS
-97.7
-210
-57.5
-218.8
-187.7
-96.6
0.0
Latent Heat
of Fusion Temperature, Density
hif, kJ/kg
°C
p, kg/m³
322.4
28
126
80.3
109
108
181.1
200.6
59.5
105.7
11.4
58.4
99.2
25.3
180.7
13.7
80.0
333.7
-33.3
-20
0
25
-185.6
20
20
-0.5
0
25
20
20
20
-268.9
-252.8
-11.7
20
25
-161.5
-100
25
-195.8
- 160
20
25
- 183
20
-42.1
0
50
-50
-26.1
0
25
0
25
Liquid Properties
50 75 00
682
665
639
602
1394
879
1150
601
298
783
789
1109
1261
146.2
70.7
593.8
820
13,560
423
301
787
809
596
703
910
1141
640
581
529
449
1443
1374
1295
1207
1000
997
988
975
958
Specific
Heat
Cp, kJ/kg.K
4.43
4.52
4.60
4.80
1.14
1.72
3.11
2.31
0.59
2.46
2.84
2.84
2.32
22.8
10.0
2.28
2.00
0.139
3.49
5.79
2.55
2.06
2.97
2.10
1.80
1.71
2.0
2.25
2.53
3.13
1.23
1.27
1.34
1.43
4.22
4.18
4.18
4.19
4.22
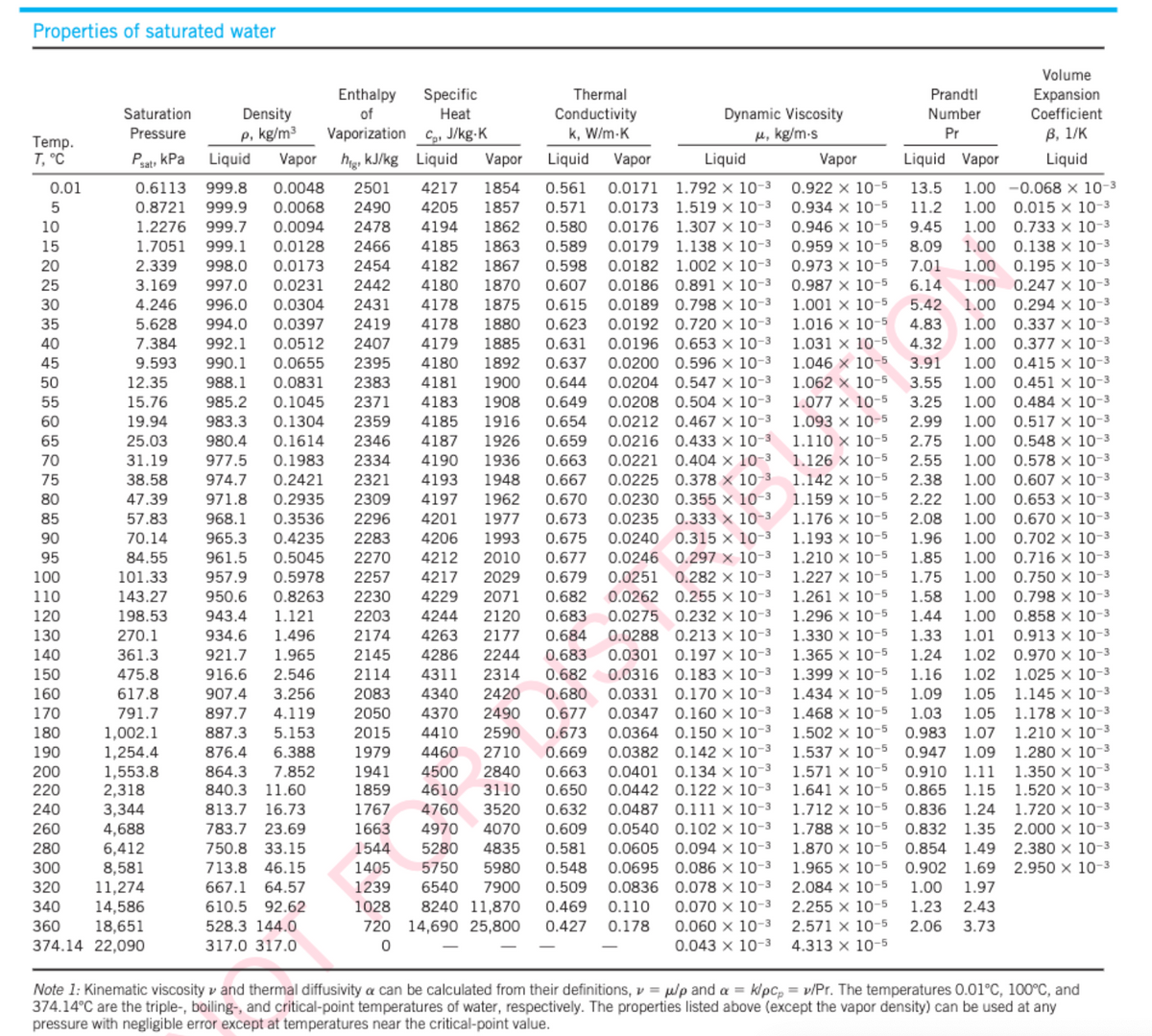
Transcribed Image Text:Properties of saturated water
Temp.
T, °C
0.01
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
80
85
90
95
100
110
120
130
140
150
160
170
180
190
200
220
240
260
280
300
320
340
360
Enthalpy Specific
of
Heat
Density
p, kg/m³
Thermal
Conductivity
k, W/m.K
Vaporization C, J/kg.K
Liquid Vapor
Liquid Vapor
Liquid
9.593
4190
1936
1948
971.8 0.2935 2309
hig, kJ/kg Liquid Vapor
0.6113 999.8 0.0048 2501 4217 1854 0.561 0.0171 1.792 x 10-3
0.8721 999.9 0.0068 2490 4205 1857 0.571 0.0173 1.519 x 10-3
1.2276 999.7 0.0094 2478 4194 1862 0.580 0.0176 1.307 x 10-3
1.7051 999.1 0.0128 2466 4185 1863 0.589 0.0179 1.138 x 10-³
2.339 998.0 0.0173 2454 4182 1867 0.598 0.0182 1.002 x 10-³
3.169 997.0 0.0231 2442 4180 1870 0.607 0.0186 0.891 x 10-³
4.246 996.0 0.0304 2431 4178 1875 0.615 0.0189 0.798 x 10-³
5.628 994.0 0.0397 2419
4178 1880 0.623 0.0192 0.720 x 10-³
7.384 992.1 0.0512 2407 4179 1885 0.631 0.0196 0.653 x 10-3
990.1 0.0655 2395 4180 1892 0.637 0.0200 0.596 x 10-³
988.1 0.0831 2383 4181 1900 0.644 0.0204 0.547 x 10-3
985.2 0.1045 2371 4183 1908 0.649 0.0208 0.504 x 10-3
983.3 0.1304 2359 4185 1916 0.654 0.0212 0.467 x 10-³
980.4 0.1614 2346 4187 1926 0.659 0.0216 0.433 x 10-3
977.5 0.1983
2334
0.663 0.0221 0.404 x 10-3
974.7 0.2421 2321 4193
0.667 0.0225 0.378 x 10-3
4197 1962 0.670 0.0230 0.355 x 10-³
968.1 0.3536 2296 4201 1977 0.673 0.0235 0.333 x 10-³
965.3 0.4235 2283 4206 1993 0.675 0.0240 0.315 x 10-3
961.5 0.5045 2270 4212 2010 0.677 0.0246 0.297 x 10-³
957.9 0.5978 2257 4217 2029 0.679 0.0251 0.282 x 10-3
950.6 0.8263 2230 4229 2071 0.682 0.0262 0.255 x 10-3
2203
4244 2120
0.683 0.0275 0.232 x 10-³
2174 4263 2177 0.684 0.0288 0.213 x 10-3
2145 4286 2244 0.683 0.0301 0.197 X 10-³
2114 4311 2314 0.682 0.0316 0.183 x 10-3
2083 4340 2420 0.680 0.0331 0.170 x 10-³
2050 4370 2490 0.677 0.0347 0.160 x 10-³
2015 4410 2590 0.673 0.0364 0.150 x 10-³
1979 4460 2710 0.669 0.0382 0.142 x 10-³
1941 4500 2840 0.663 0.0401 0.134 x 10-³
1859 4610 3110 0.650 0.0442 0.122 x 10-3
1767 4760 3520 0.632 0.0487 0.111 x 10-3
1663 4970 4070 0.609 0.0540 0.102 x 10-³
1544 5280
4835 0.581 0.0605 0.094 x 10-3
1405 5750 5980 0.548 0.0695 0.086 x 10-3
1239 6540 7900 0.509 0.0836 0.078 x 10-³
1028 8240 11,870 0.469 0.110 0.070 x 10-3
720 14,690 25,800 0.427 0.178 0.060 x 10-3
0
0.043 x 10-3
943.4 1.121
934.6 1.496
921.7 1.965
916.6 2.546
907.4 3.256
897.7 4.119
887.3 5.153
876.4 6.388
864.3 7.852
840.3 11.60
813.7 16.73
783.7 23.69
750.8 33.15
713.8 46.15
667.1 64.57
610.5 92.62
528.3 144.0
317.0 317.0
Saturation
Pressure
Psat kPa
12.35
15.76
19.94
25.03
31.19
38.58
47.39
57.83
70.14
84.55
101.33
143.27
198.53
270.1
361.3
475.8
617.8
791.7
1,002.1
1,254.4
1,553.8
2,318
3,344
4,688
6,412
8,581
11,274
14,586
18,651
374.14 22,090
Dynamic Viscosity
μ, kg/m-s
Prandtl
Number
Pr
Vapor
Liquid Vapor
0.922 x 10-5
0.934 x 10-5
0.946 x 10-5
13.5
11.2
9.45
8.09
7.01
6.14
5.42
1.00
1.00
1.00
1.00
1.00
0.959 x 10-5
0.973 x 10-5
0.987 x 10-5
1.001 x 10-5
1.016 x 10-5
1.031 x 10-5
1.046 x 10-5
1.062 x 10-5
1.077 x 10-5
1.093 x 10-5
1.110 x 10-5
1.126 x 10-5
1.142 x 10-5
1.159 x 10-5
1.176 x 10-5
1.193 x 10-5
1.210 x 10-5
1.227 x 10-5
1.261 x 10-5
1.296 x 10-5
4.83
1.00
4.32 1.00
3.91 1.00
3.55 1.00
3.25 1.00
2.99 1.00
2.75 1.00
2.55 1.00
2.38 1.00
2.22 1.00
2.08 1.00
1.96 1.00
1.85
1.75
1.58
1.00
1.00
1.00
1.00
1.330 x 10-5
1.44
1.33
1.24
1.01
1.02
1.365 x 10-5
1.399 x 10-5
1.434 x 10-5
1.16
1.02
1.05
1.09
1.468 x 10-5 1.03 1.05
1.502 x 10-5 0.983 1.07
1.537 x 10-5 0.947 1.09
1.571 x 10-5 0.910 1.11
1.641 x 10-5 0.865 1.15
1.712 x 10-5 0.836 1.24
1.788 x 10-5 0.832 1.35
1.870 x 10-5 0.854
1.965 x 10-5 0.902
2.084 x 10-5
2.255 x 10-5
2.571 x 10-5
4.313 x 10-5
1.49
1.69
1.97
2.43
3.73
1.00
1.23
2.06
Volume
Expansion
Coefficient
B, 1/K
Liquid
1.00 -0.068 x 10-3
1.00
0.015 x 10-3
0.733 x 10-3
0.138 x 10-3
0.195 x 10-3
0.247 x 10-3
0.294 x 10-3
0.337 x 10-3
0.377 x 10-3
0.415 x 10-3
0.451 x 10-3
0.484 x 10-3
0.517 x 10-3
0.548 x 10-3
0.578 x 10-³
0.607 x 10-³
0.653 x 10-3
0.670 x 10-3
0.702 x 10-³
0.716 x 10-3
0.750 x 10-³
0.798 x 10-3
0.858 x 10-3
0.913 x 10-3
0.970 x 10-³
1.025 x 10-³
1.145 x 10-3
1.178 x 10-3
1.210 x 10-³
1.280 x 10-3
1.350 x 10-3
1.520 x 10-3
1.720 x 10-3
2.000 x 10-3
2.380 x 10-3
2.950 x 10-3
Note 1: Kinematic viscosity and thermal diffusivity a can be calculated from their definitions, p/p and a = k/pcp=
= v/Pr. The temperatures 0.01°C, 100°C, and
374.14°C are the triple-, boiling-, and critical-point temperatures of water, respectively. The properties listed above (except the vapor density) can be used at any
pressure with negligible error except at temperatures near the critical-point value.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning