A 3.48 liter glass bottle is full of oil. Assume it contains 3.48 Lof oil. The density of oil is 1.38 g/mL. The glass bottle itself-has mass of 382 grams. What is the weight of bottle plus oil in pounds? This is similar to question 11 in 'some extra questions' in module. You need to write your answer in the box with two numbers past the decimal.
A 3.48 liter glass bottle is full of oil. Assume it contains 3.48 Lof oil. The density of oil is 1.38 g/mL. The glass bottle itself-has mass of 382 grams. What is the weight of bottle plus oil in pounds? This is similar to question 11 in 'some extra questions' in module. You need to write your answer in the box with two numbers past the decimal.
Chapter1: Matter, Measurements, And Calculations
Section: Chapter Questions
Problem 1.41E
Related questions
Question
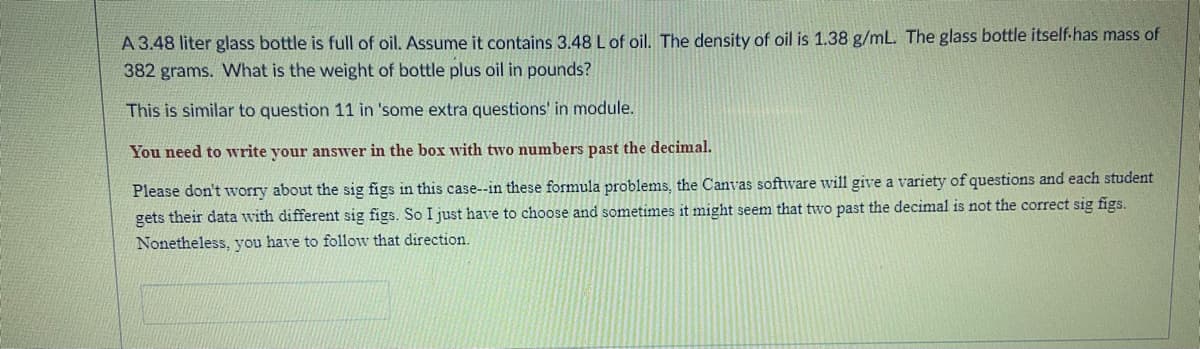
Transcribed Image Text:A 3.48 liter glass bottle is full of oil. Assume it contains 3.48 Lof oil. The density of oil is 1.38 g/mL. The glass bottle itself-has mass of
382 grams. What is the weight of bottle plus oil in pounds?
This is similar to question 11 in 'some extra questions' in module.
You need to write your answer in the box with two numbers past the decimal.
Please don't worry about the sig figs in this case--in these formula problems, the Canvas software will give a variety of questions and each student
gets their data with different sig figs. So I just have to choose and sometimes it might seem that two past the decimal is not the correct sig figs.
Nonetheless, you have to follow that direction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning