Thermometer Ignition wires heat Stirrer sample A bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter. Water In the laboratory a student burns a 1.02-g sample of dimethyl oxalate (C4H6O4) in a bomb calorimeter containing 1140. g of water. The temperature increases from 25.90 °C to 28.50 °C. The heat capacity of water is 4.184 J g °C. The molar heat of combustion is -1675 kJ per mole of dimethyl oxalate CAH6O4(s)7/2 O2(g) >4 CO2(g)3 H2O(l) Energy Calculate the heat capacity of the calorimeter. Insulated outside chamber Sample dish Burning sample Steel bomb heat capacity of calorimeter J/OC Combustion (bomb) calorimeter.
Thermometer Ignition wires heat Stirrer sample A bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter. Water In the laboratory a student burns a 1.02-g sample of dimethyl oxalate (C4H6O4) in a bomb calorimeter containing 1140. g of water. The temperature increases from 25.90 °C to 28.50 °C. The heat capacity of water is 4.184 J g °C. The molar heat of combustion is -1675 kJ per mole of dimethyl oxalate CAH6O4(s)7/2 O2(g) >4 CO2(g)3 H2O(l) Energy Calculate the heat capacity of the calorimeter. Insulated outside chamber Sample dish Burning sample Steel bomb heat capacity of calorimeter J/OC Combustion (bomb) calorimeter.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 80E: Ethanol, C2H5OH, is used as a fuel for motor vehicles, particularly in Brazil. (a) Write the...
Related questions
Question
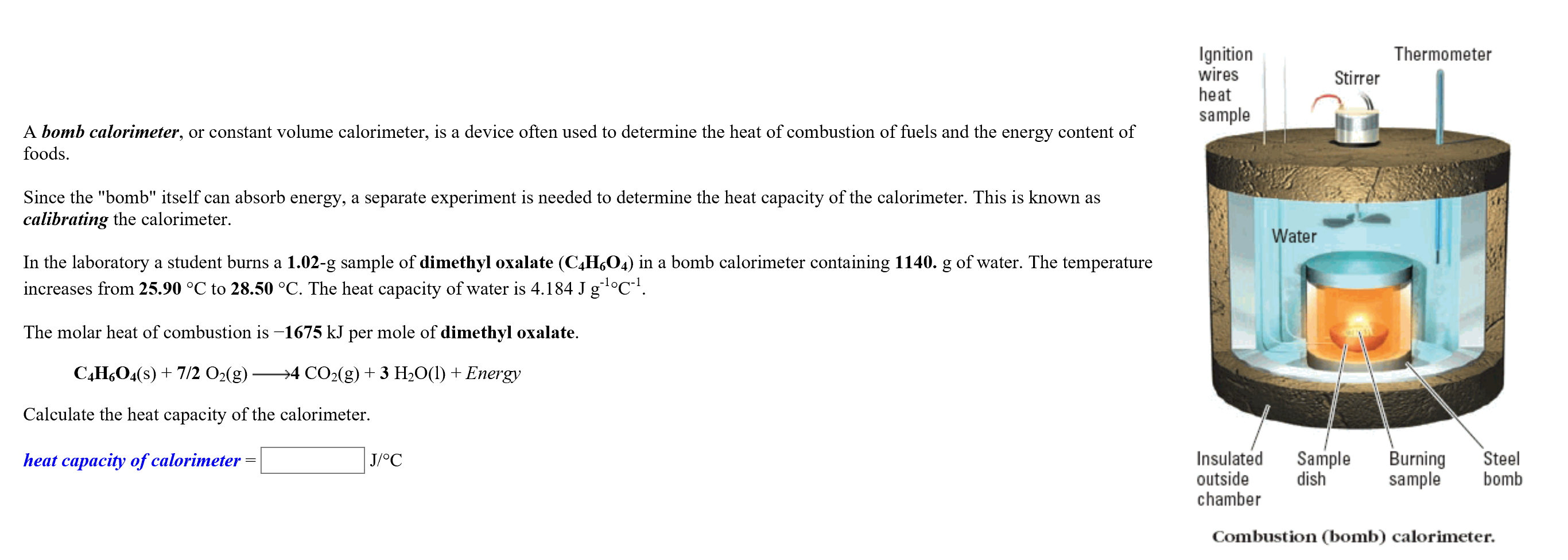
Transcribed Image Text:Thermometer
Ignition
wires
heat
Stirrer
sample
A bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of
foods
Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as
calibrating the calorimeter.
Water
In the laboratory a student burns a 1.02-g sample of dimethyl oxalate (C4H6O4) in a bomb calorimeter containing 1140. g of water. The temperature
increases from 25.90 °C to 28.50 °C. The heat capacity of water is 4.184 J g °C.
The molar heat of combustion is -1675 kJ per mole of dimethyl oxalate
CAH6O4(s)7/2 O2(g)
>4 CO2(g)3 H2O(l) Energy
Calculate the heat capacity of the calorimeter.
Insulated
outside
chamber
Sample
dish
Burning
sample
Steel
bomb
heat capacity of calorimeter
J/OC
Combustion (bomb) calorimeter.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning