(a) A 1:1 (i.e. racemic) mixture of the two enantiomers of a chiral medicine was subjected to HPLC on a column with a chiral stationary phase of length 25.0 cm and internal diameter 4.6 mm. Elution was done with hexane-ethanol-diethylamine at a flow rate of 1 mL/min and peaks (listed in Table 1) were detected by measuring UV absorbance at λ = 220 nm. Peak assignment Retention time / min Width at peak of Unretained solvent First enantiomer Second enantiomer 3.05 5.62 6.44 base (W) / min Not determined 0.45 0.64 Area % 3.65 50.12 49.88 Table 1. Peaks observed during HPLC analysis of a racemic sample of chiral medicine
(a) A 1:1 (i.e. racemic) mixture of the two enantiomers of a chiral medicine was subjected to HPLC on a column with a chiral stationary phase of length 25.0 cm and internal diameter 4.6 mm. Elution was done with hexane-ethanol-diethylamine at a flow rate of 1 mL/min and peaks (listed in Table 1) were detected by measuring UV absorbance at λ = 220 nm. Peak assignment Retention time / min Width at peak of Unretained solvent First enantiomer Second enantiomer 3.05 5.62 6.44 base (W) / min Not determined 0.45 0.64 Area % 3.65 50.12 49.88 Table 1. Peaks observed during HPLC analysis of a racemic sample of chiral medicine
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.25P
Related questions
Question
i) Calculate the selectivity factor, , for the separation of the two enantiomers.
(ii) Calculate the resolution, RS, of the pair of peaks corresponding to the two enantiomers
of the medicine.
(iii) Suggest two modifications that could be made to the analytical method described
above with the aim of increasing the observed value of RS. Briefly explain your choices
and indicate any likely disadvantages of your proposed modifications.
(iv) A non-racemic sample of the medicine gave peaks with retention times of 6.42 min
(98.8% relative area) and 5.61 min (1.2% relative area). Calculate the enantiomeric
excess of this second sample, stating any assumptions that you make.
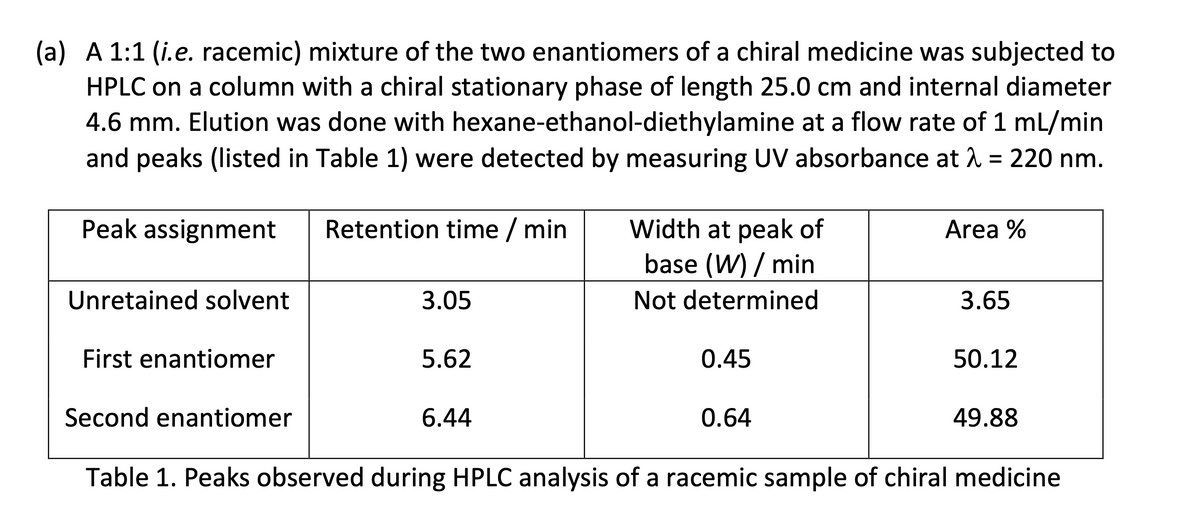
Transcribed Image Text:(a) A 1:1 (i.e. racemic) mixture of the two enantiomers of a chiral medicine was subjected to
HPLC on a column with a chiral stationary phase of length 25.0 cm and internal diameter
4.6 mm. Elution was done with hexane-ethanol-diethylamine at a flow rate of 1 mL/min
and peaks (listed in Table 1) were detected by measuring UV absorbance at λ = 220 nm.
Peak assignment
Retention time / min
Width at peak of
Unretained solvent
First enantiomer
Second enantiomer
3.05
5.62
6.44
base (W) / min
Not determined
0.45
0.64
Area %
3.65
50.12
49.88
Table 1. Peaks observed during HPLC analysis of a racemic sample of chiral medicine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning