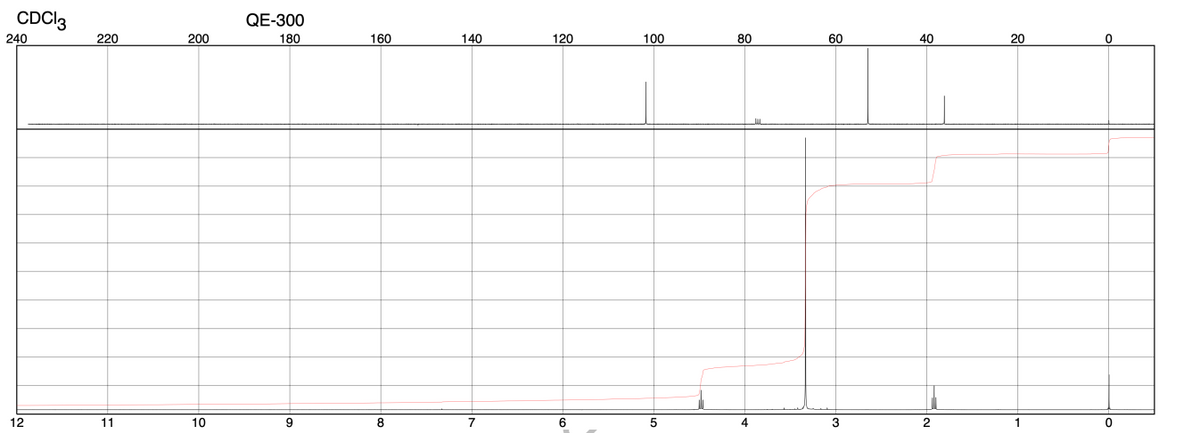
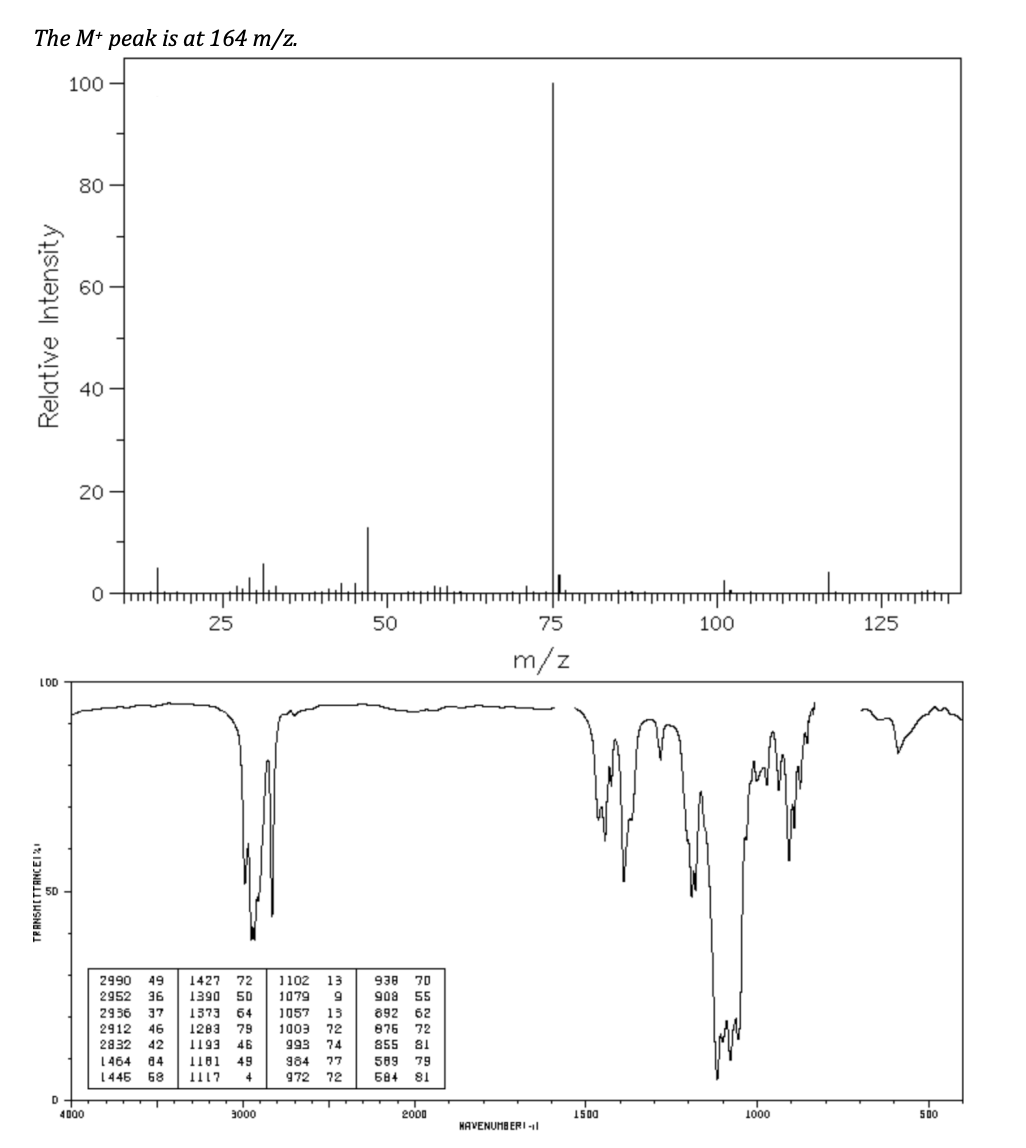
Based on the spectra provided, draw the structure. Label each unique carbon and hydrogen with the letters A, B, C… for use in assigning NMR peaks. fill in the data table assigning peaks in each spectrum. You should assign: • All 1H NMR peaks (do not worry about the peaks for CDCl3 at 7.26 ppm or the peak for TMS at 0 ppm) • Significant IR peaks above 1600 cm
Based on the spectra provided, draw the structure. Label each unique carbon and hydrogen with the letters A, B, C… for use in assigning NMR peaks. fill in the data table assigning peaks in each spectrum. You should assign:
• All 1H NMR peaks (do not worry about the peaks for CDCl3 at 7.26 ppm or the peak for TMS at 0 ppm)
• Significant IR peaks above 1600 cm
Example of what the table should include (imagine the structure of ethanol is drawn with the CH3 hydrogens labeled as A, the CH2 hydrogens labeled as B, and the OH hydrogen labeled as C) :
| hydrogen | proton chemical shift | integration | splitting pattern | couples to.. |
| A | 1.2 | 3 | triplet | B |
| B | 3.7 | 2 | quartet | A |
| C | 2.6 | 1 | singlet | - |
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

can you also include another table with ir data, where one column is wavenumber (cm^-1) and the other column is






