A ALEKS Hailemariam Mengisti X Ce www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P... E Initlal Knowledge Check Question Decide whether each pair of elements in the table below will form an ionic compound. If th formed in the spaces provided. Forms ionic compound? empirical formula of ionic element #1 element #2 name of ionic compound compound bromine oxygen yes no rubidium barium yes O no MgO magnesium oxide magnesium O yes no oxygen jodine rubidium yes no I Don't Know Submit
A ALEKS Hailemariam Mengisti X Ce www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P... E Initlal Knowledge Check Question Decide whether each pair of elements in the table below will form an ionic compound. If th formed in the spaces provided. Forms ionic compound? empirical formula of ionic element #1 element #2 name of ionic compound compound bromine oxygen yes no rubidium barium yes O no MgO magnesium oxide magnesium O yes no oxygen jodine rubidium yes no I Don't Know Submit
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 68P: Mixing SbCl3 and GaCl3 in a 1:1 molar ratio (using liquid sulfur dioxide as a solvent) gives a solid...
Related questions
Question
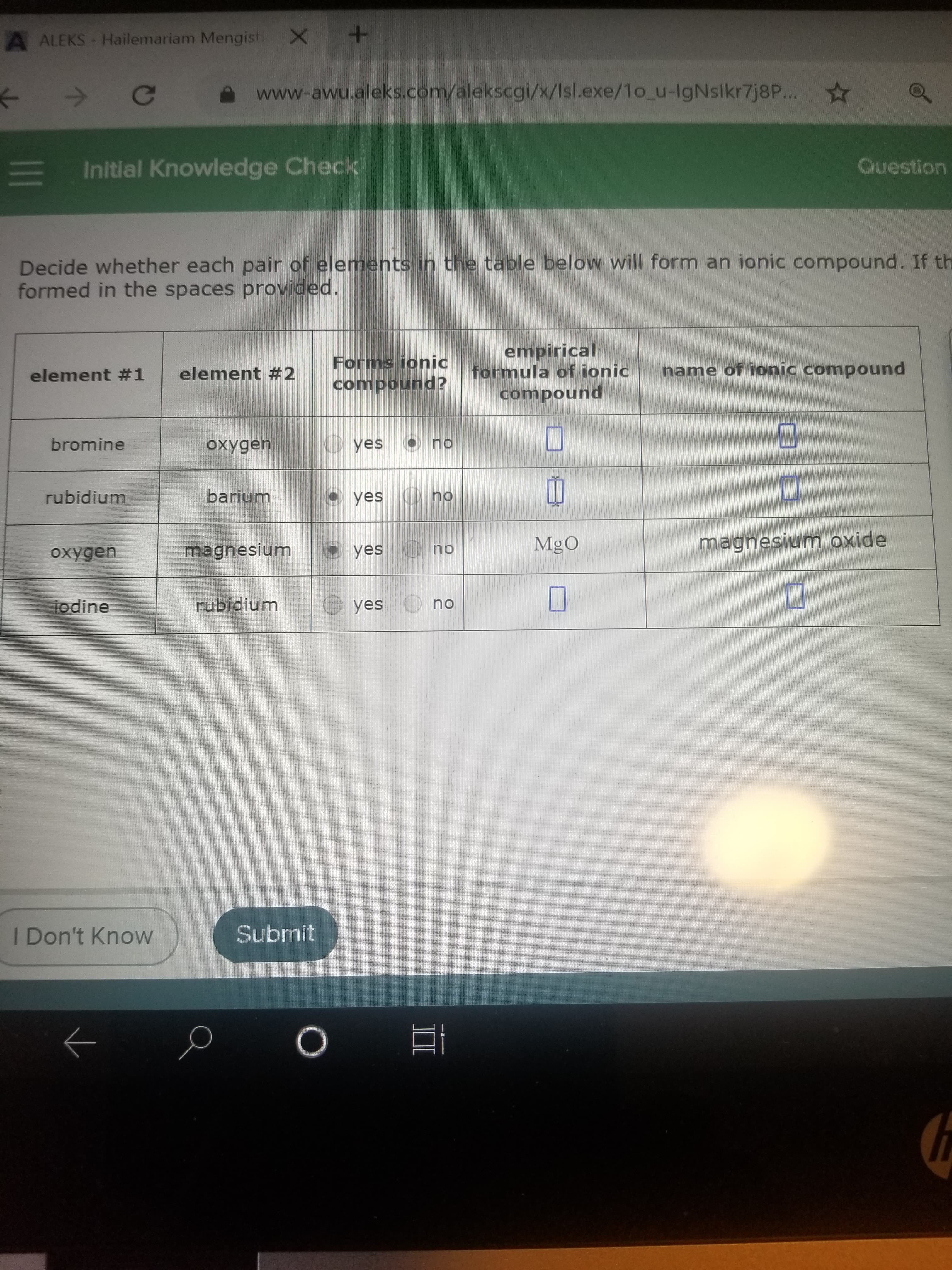
Transcribed Image Text:A ALEKS Hailemariam Mengisti X
Ce
www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P...
E Initlal Knowledge Check
Question
Decide whether each pair of elements in the table below will form an ionic compound. If th
formed in the spaces provided.
Forms ionic
compound?
empirical
formula of ionic
element #1
element #2
name of ionic compound
compound
bromine
oxygen
yes
no
rubidium
barium
yes O no
MgO
magnesium oxide
magnesium
O yes
no
oxygen
jodine
rubidium
yes
no
I Don't Know
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
