Rank the attached compounds in order of increasing acidity.
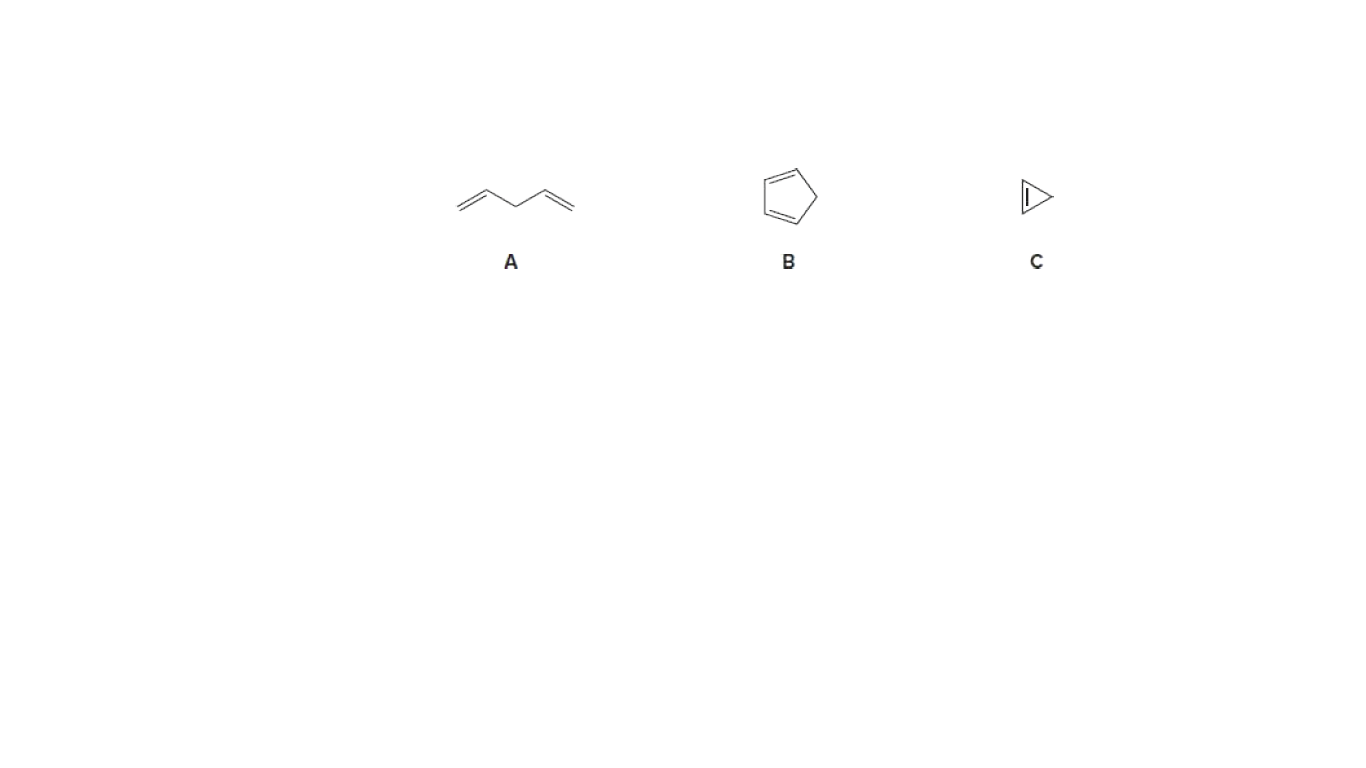
Acidity of any molecule depends on the stability of the anion formed after removing the most acidic H from the molecule.
More is the stability of the anion, more will be the acidity of the molecule.
The anions that will form after removing the acidic H from the above molecules are as shown below.
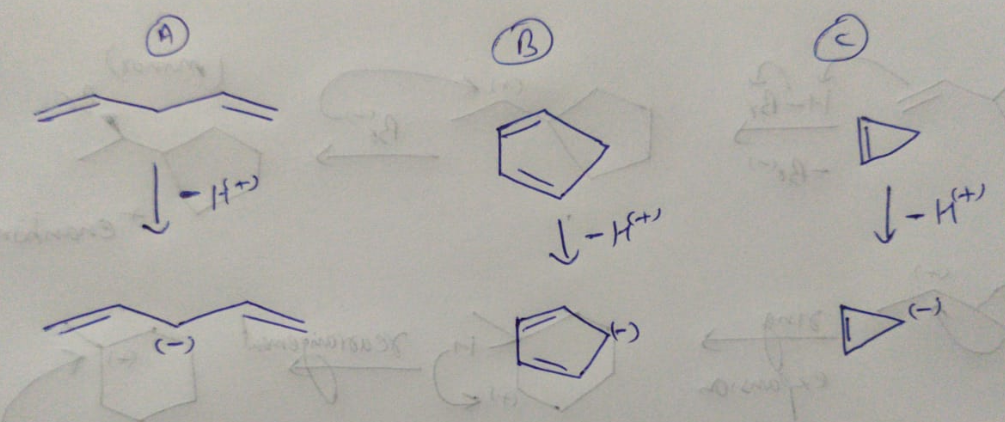
The factors that confer aromaticity to an organic molecule are
1) The molecule should be having a planar ring
2) All the atoms present in the ring should be in conjugation with each other.
3) There should be 4n+2 pi electrons in inside the ring where n can be any whole number.
If the number of pi electrons is 4n instead of 4n +2 with above rules, then it confers that the molecule is anti-aromatic.
And if the above rules are not satisfied, then the compound is non-aromatic.
NOTE : If there is a single aromatic ring also present in the compound, then the whole compound is considered as aromatic.
Aromaticity of any molecule means the extra stability of the molecule due to being aromatic.
And anti-aromaticity of any molecule means extra destability of the molecule due to being anti-aromatic.
Step by step
Solved in 4 steps with 1 images









