A beautiful red mineral, rhodochriosite, is manganese (I1) carbonate (MNCO3). a. Write balanced molecular equation for the reaction of the mineral with hydrochloric acid (HCI). 1. b. Name the products. What are the phases of each product? d. Write the Net lonic Equation. C. e. What qualitative observations would indicate a chemical change occurred?
A beautiful red mineral, rhodochriosite, is manganese (I1) carbonate (MNCO3). a. Write balanced molecular equation for the reaction of the mineral with hydrochloric acid (HCI). 1. b. Name the products. What are the phases of each product? d. Write the Net lonic Equation. C. e. What qualitative observations would indicate a chemical change occurred?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question

Transcribed Image Text:A beautiful red mineral, rhodochriosite, is manganese (I1) carbonate (MNCO3).
a. Write balanced molecular equation for the reaction of the mineral with hydrochloric acid (HCI).
1.
b. Name the products.
What are the phases of each product?
d. Write the Net lonic Equation.
C.
e. What qualitative observations would indicate a chemical change occurred?
Expert Solution
Step 1
Hello. Since your question has multiple sub-parts, we will solve first three sub-parts for you. If you want remaining sub-parts to be solved, then please resubmit the whole question and specify those sub-parts you want us to solve.
Step 2
(a) The balanced molecular equation for the reaction of manganese(II) carbonate with hydrochloric acid is:
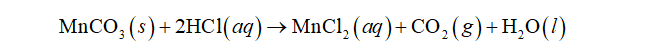
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning