A mixture of MgCl, and inert material is analyzed to determine the Mg content. First the mixture is dissolved in water. Then all of the chloride ion is precipitated as AgCl by the addition of an excess of silver nitrate. MgCly(aq) + 2A9NO3(aq) 2AGCI(s) + Mg(NO3)2(aq) In one experiment, a 0.5770 g sample of the mixture resulted in 1.3717 g of AgCI. Determine the percent (by mass) of Mg in the mixture. % Mg
A mixture of MgCl, and inert material is analyzed to determine the Mg content. First the mixture is dissolved in water. Then all of the chloride ion is precipitated as AgCl by the addition of an excess of silver nitrate. MgCly(aq) + 2A9NO3(aq) 2AGCI(s) + Mg(NO3)2(aq) In one experiment, a 0.5770 g sample of the mixture resulted in 1.3717 g of AgCI. Determine the percent (by mass) of Mg in the mixture. % Mg
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter6: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 108AE
Related questions
Question
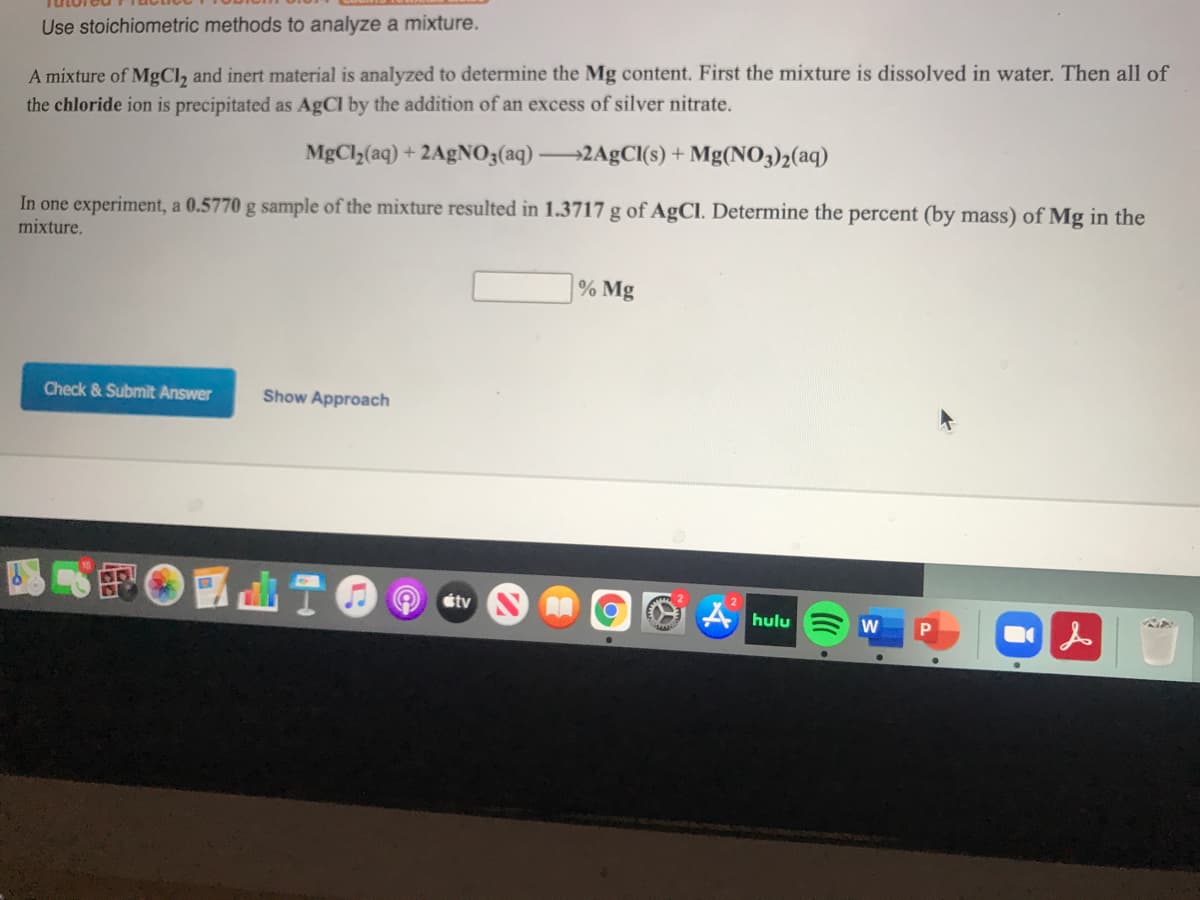
Transcribed Image Text:Use stoichiometric methods to analyze a mixture.
A mixture of MGCI, and inert material is analyzed to determine the Mg content. First the mixture is dissolved in water. Then all of
the chloride ion is precipitated as AgCl by the addition of an excess of silver nitrate.
MgCl,(aq) + 2A£NO3(aq) 2A9CI(s) + Mg(NO3)2(aq)
In one experiment, a 0.5770 g sample of the mixture resulted in 1.3717 g of AgCI. Determine the percent (by mass) of Mg in the
mixture.
% Mg
Check & Submit Answer
Show Approach
étv
A hulu
工甲盟(
Expert Solution
Step 1: Interpretation
The given reaction is a precipitation reaction in which the precipitate formed is AgCl.
Steps involved to determine % mass of Mg are:
Step 1:
we should determine number of moles of AgCl precipitated.
Step 2:
From number of moles of AgCl precipitated, number of moles of Mg can be interpreted stoichiometrically.
Step 3:
Mass of Mg can de determined from number of moles of Mg by substituting in mole formula.
Step 4:
%mass of Mg in mixture is obtained by substituting the mass of Mg in %mass formula.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning