A botanist is trying to determine the effect of light intensity on the rate of photosynthesis in aquatic plants. They create a setup as shown in the diagram to the right. The chemical equation for photosynthesis is: 6CO2(g) + 6H2O (l) → C6H12O6(s) + 602(g) a. Describe how the botanist is determining the rate of the photosynthesis reaction. cm ruler test tube water with dissolved CO2 bubbles of gas lamp 0 10 S aquatic plant b. Sketch a graph showing how you would expect the concentration of CO2 in the water to change over the course of the reaction. Concentration of CO2 (mol/L) Time (min)
A botanist is trying to determine the effect of light intensity on the rate of photosynthesis in aquatic plants. They create a setup as shown in the diagram to the right. The chemical equation for photosynthesis is: 6CO2(g) + 6H2O (l) → C6H12O6(s) + 602(g) a. Describe how the botanist is determining the rate of the photosynthesis reaction. cm ruler test tube water with dissolved CO2 bubbles of gas lamp 0 10 S aquatic plant b. Sketch a graph showing how you would expect the concentration of CO2 in the water to change over the course of the reaction. Concentration of CO2 (mol/L) Time (min)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.72PAE
Related questions
Question
Draw the graph as well
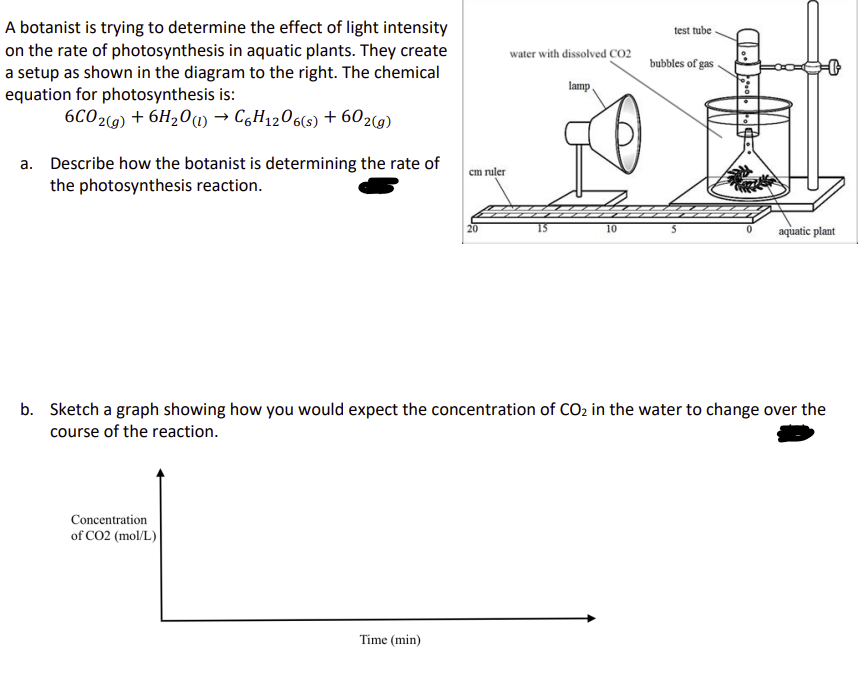
Transcribed Image Text:A botanist is trying to determine the effect of light intensity
on the rate of photosynthesis in aquatic plants. They create
a setup as shown in the diagram to the right. The chemical
equation for photosynthesis is:
6CO2(g) + 6H2O (l) → C6H12O6(s) + 602(g)
a. Describe how the botanist is determining the rate of
the photosynthesis reaction.
cm ruler
test tube
water with dissolved CO2
bubbles of gas
lamp
0
10
S
aquatic plant
b. Sketch a graph showing how you would expect the concentration of CO2 in the water to change over the
course of the reaction.
Concentration
of CO2 (mol/L)
Time (min)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax