121 (OL) Lab 3 Den... V Layout Search References Mailings Review View Help 1 2 3 5 6 I i Lynette Maina Commer 7 LE T Chem 121(OL) Lab 3: Density of Common Materials Part C Thickness of Aluminum Foil (For help understanding the calculations read the introduction and procedure) An experiment performed for Part C to measure the length, width and mass of a piece of aluminum foil gave the following data. You will use this data to solve for the thickness of aluminum foil using the equation from Part C in the introduction. Length of foil Width of foil Mass of foil 13.10 cm 9.50 cm 9.745 g Use the data in the table to determine the thickness of your aluminum foil (in cm) using the 2.6989 g/mL for the density of aluminum then convert this value to meters (m) and micrometers (um). Show your work here (use back if necessary). ons: On Accessibility: Investigate F3 F4 W cm K F5 ►11 F6 K H m um DELL F7 F8 Gu F9 Focus BB 5= 57°F Mostly sunny 4/1 F10 F11 F12 PrtScr "T
121 (OL) Lab 3 Den... V Layout Search References Mailings Review View Help 1 2 3 5 6 I i Lynette Maina Commer 7 LE T Chem 121(OL) Lab 3: Density of Common Materials Part C Thickness of Aluminum Foil (For help understanding the calculations read the introduction and procedure) An experiment performed for Part C to measure the length, width and mass of a piece of aluminum foil gave the following data. You will use this data to solve for the thickness of aluminum foil using the equation from Part C in the introduction. Length of foil Width of foil Mass of foil 13.10 cm 9.50 cm 9.745 g Use the data in the table to determine the thickness of your aluminum foil (in cm) using the 2.6989 g/mL for the density of aluminum then convert this value to meters (m) and micrometers (um). Show your work here (use back if necessary). ons: On Accessibility: Investigate F3 F4 W cm K F5 ►11 F6 K H m um DELL F7 F8 Gu F9 Focus BB 5= 57°F Mostly sunny 4/1 F10 F11 F12 PrtScr "T
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 43GQ: Hexane (C6H14, density = 0.766 g/cm3), perfluoro-hexane (C6F14, density = 1.669 g/cm3), and water...
Related questions
Question
Transcribed Image Text:121 (OL) Lab 3 Den... V
Layout
Search
References
Mailings
Review
View
Help
1
2
3
5
6 I
i
Lynette Maina
Commer
7
LE
T
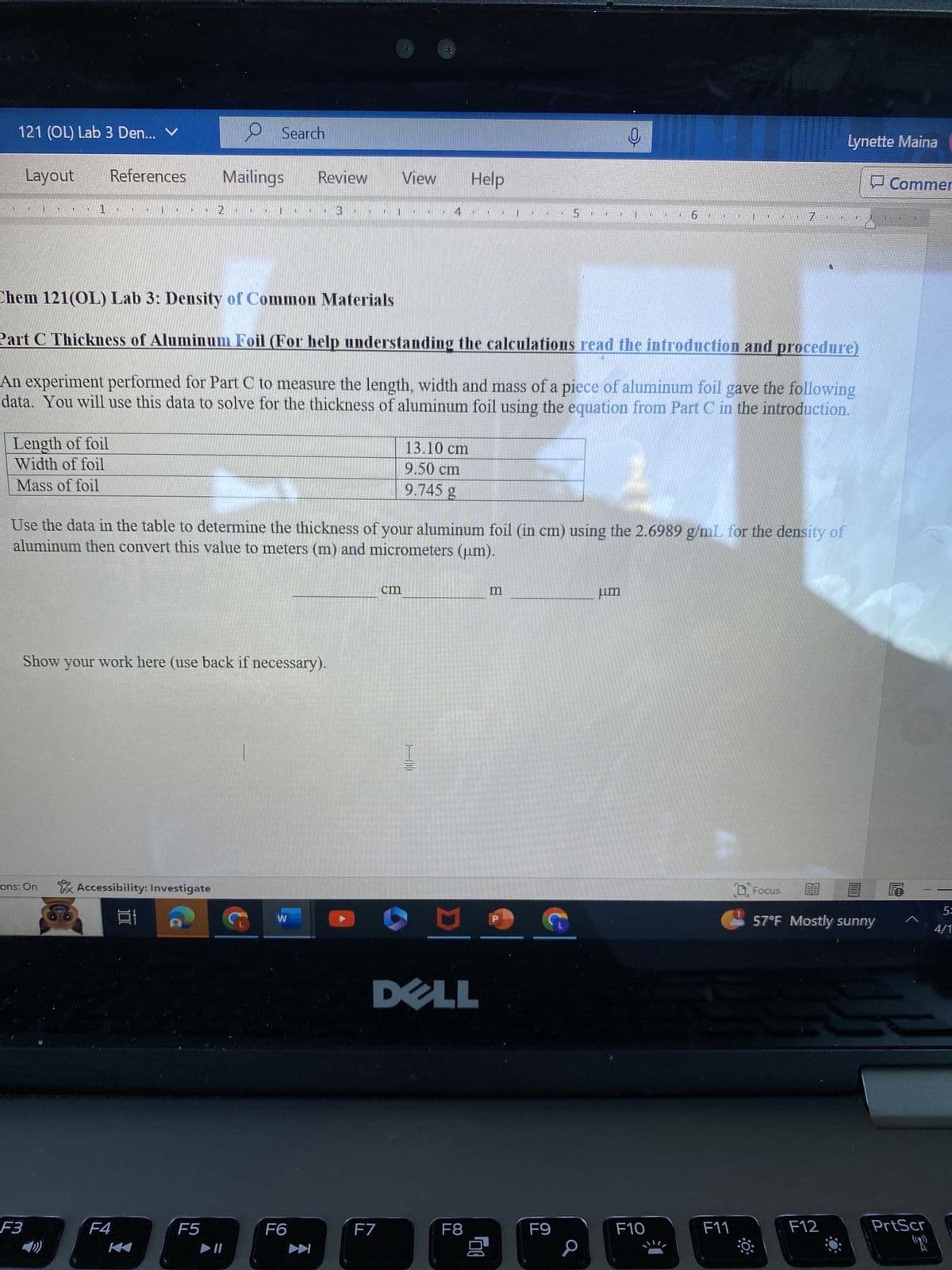
Chem 121(OL) Lab 3: Density of Common Materials
Part C Thickness of Aluminum Foil (For help understanding the calculations read the introduction and procedure)
An experiment performed for Part C to measure the length, width and mass of a piece of aluminum foil gave the following
data. You will use this data to solve for the thickness of aluminum foil using the equation from Part C in the introduction.
Length of foil
Width of foil
Mass of foil
13.10 cm
9.50 cm
9.745 g
Use the data in the table to determine the thickness of your aluminum foil (in cm) using the 2.6989 g/mL for the density of
aluminum then convert this value to meters (m) and micrometers (um).
Show your work here (use back if necessary).
ons: On
Accessibility: Investigate
F3
F4
W
cm
K
F5
►11
F6
K
H
m
um
DELL
F7
F8
Gu
F9
Focus
BB
5=
57°F Mostly sunny
4/1
F10
F11
F12
PrtScr
"T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning