A buffer solution is prepared by dissolving 0.200 mol of NaH 2PO 4 and 0.100 mol of NaOH in enough water to make 1.00 L of solution. What is the pH of the H 2PO 4/HPO 42- buffer if the K a2 = 6.2 x 10 -8? 7.51 O7.71 7.21 6.91
A buffer solution is prepared by dissolving 0.200 mol of NaH 2PO 4 and 0.100 mol of NaOH in enough water to make 1.00 L of solution. What is the pH of the H 2PO 4/HPO 42- buffer if the K a2 = 6.2 x 10 -8? 7.51 O7.71 7.21 6.91
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 46E: An aqueous solution contains dissolved C6H5NH3Cl and C6H5NH2. The concentration of C6H5NH2 is 0.50 M...
Related questions
Question
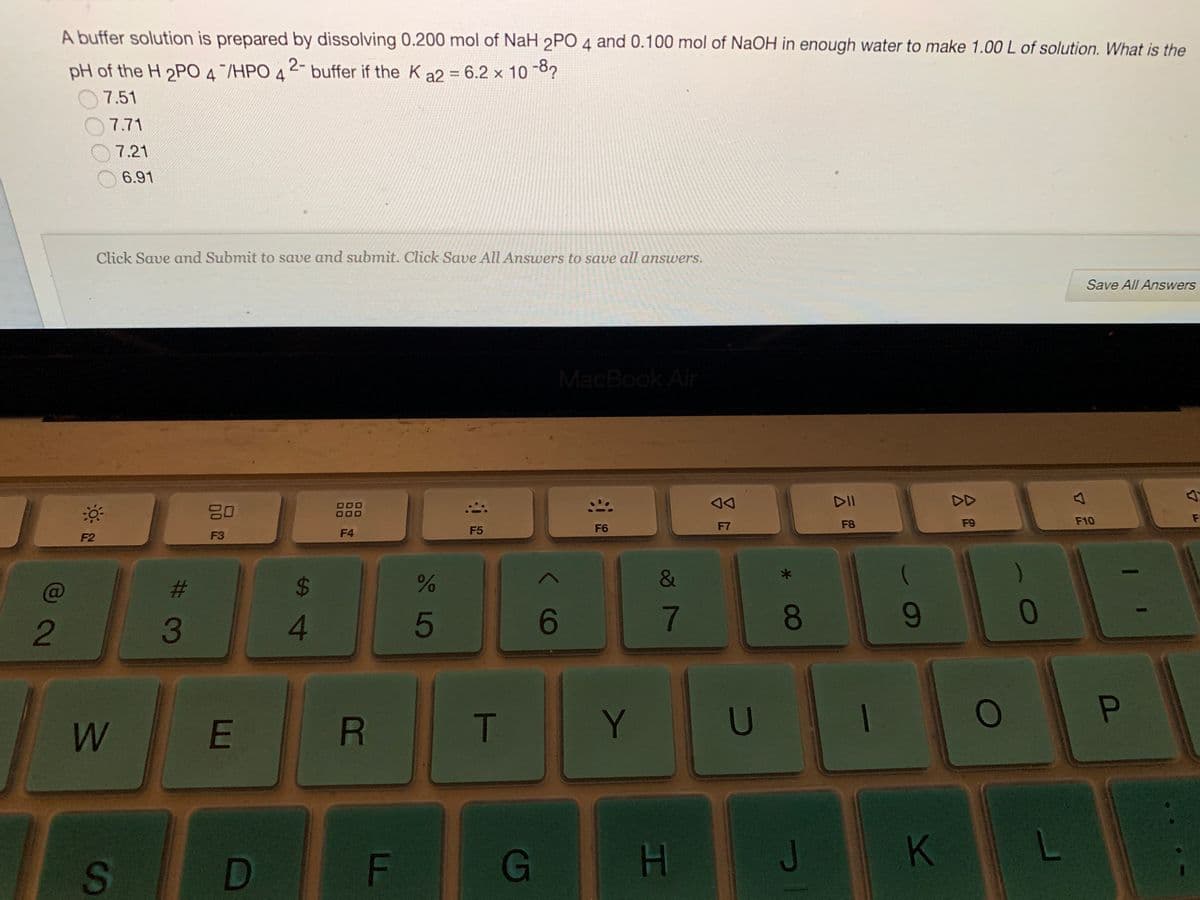
Transcribed Image Text:A buffer solution is prepared by dissolving 0.200 mol of NaH 2PO 4 and 0.100 mol of NaOH in enough water to make 1.00 L of solution. What is the
2-
pH of the H 2PO 4/HPO 4
buffer if the K a2 = 6.2 x 10 8?
7.51
7.71
7.21
6.91
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Save All Answers
MacBook Air
DD
000
000
F10
F
F6
F7
F8
F9
F3
F4
F5
F2
)
C@
$4
2
4.
5
6
7.
8.
9-
T
Y.
U
Pn
W
G
J
K
S
HI
LL
R
%# 3m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning