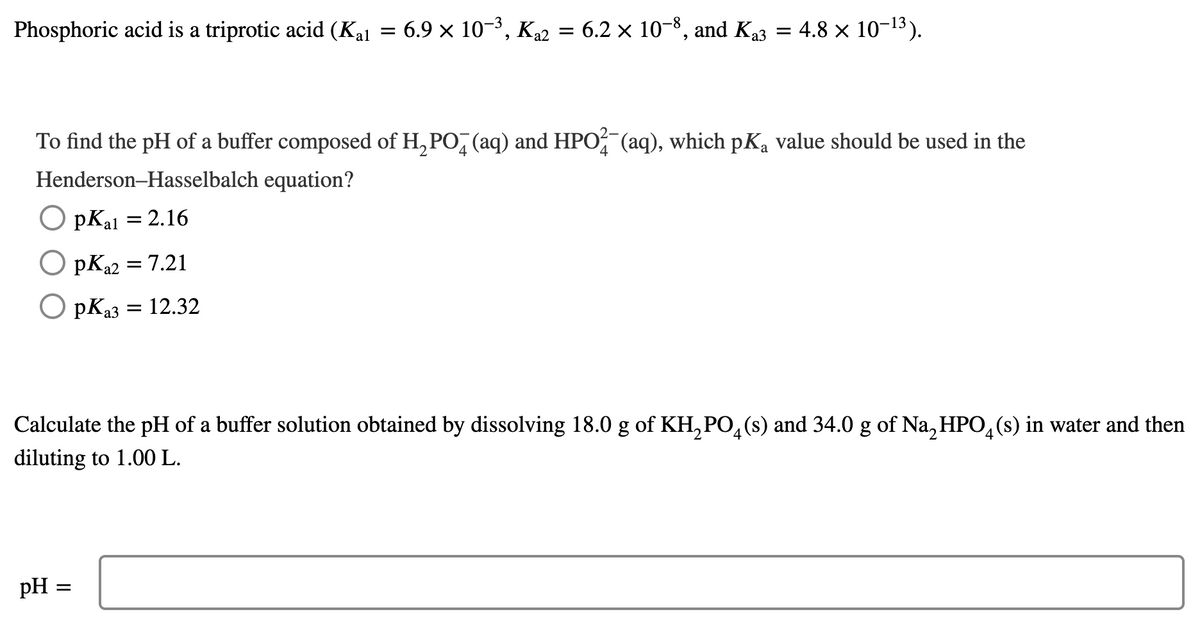
Phosphoric acid is a triprotic acid (Kal 6.9 x 10-3, K2 = 6.2 × 10-8, and Ka3 = 4.8 x 10-13). To find the pH of a buffer composed of H, PO, (aq) and HPO (aq), which pKa value should be used in the Henderson-Hasselbalch equation? pKal = 2.16 pKa2 = 7.21 O pKa3 = 12.32 %3D Calculate the pH of a buffer solution obtained by dissolving 18.0 g of KH,PO,(s) and 34.0 g of Na, HPO,(s) in water and then diluting to 1.00 L. pH =
Phosphoric acid is a triprotic acid (Kal 6.9 x 10-3, K2 = 6.2 × 10-8, and Ka3 = 4.8 x 10-13). To find the pH of a buffer composed of H, PO, (aq) and HPO (aq), which pKa value should be used in the Henderson-Hasselbalch equation? pKal = 2.16 pKa2 = 7.21 O pKa3 = 12.32 %3D Calculate the pH of a buffer solution obtained by dissolving 18.0 g of KH,PO,(s) and 34.0 g of Na, HPO,(s) in water and then diluting to 1.00 L. pH =
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 15.BCP
Related questions
Question
Transcribed Image Text:Phosphoric acid is a triprotic acid (Kal = 6.9 × 10-3, K22 = 6.2 × 10-8, and K3 = 4.8 × 10-13).
= 4.8 x 10-13).
To find the pH of a buffer composed of H, PO, (aq) and HPO (aq), which pKa value should be used in the
Henderson-Hasselbalch equation?
O pKal = 2.16
pK22 = 7.21
O pKa3 =
12.32
Calculate the pH of a buffer solution obtained by dissolving 18.0 g of KH, PO, (s) and 34.0 g of Na, HPO, (s) in water and then
4.
diluting to 1.00 L.
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning