- (a) Calculate the standard free energy change as a pair of electrons is trans- ferred from succinate to molecular oxygen in the mitochondrial respira- tory chain. (b) Based on your answer in part a, calculate the maximum number of protons that could be pumped out of the matrix into the intermembrane space as these electrons are passed to oxygen. Assume 25 °C, ApH = 1.4; Aựt = 0.175 V (matrix negative). (c) At which site(s) are these protons pumped?
- (a) Calculate the standard free energy change as a pair of electrons is trans- ferred from succinate to molecular oxygen in the mitochondrial respira- tory chain. (b) Based on your answer in part a, calculate the maximum number of protons that could be pumped out of the matrix into the intermembrane space as these electrons are passed to oxygen. Assume 25 °C, ApH = 1.4; Aựt = 0.175 V (matrix negative). (c) At which site(s) are these protons pumped?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.104QE
Related questions
Question
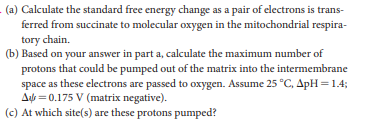
Transcribed Image Text:- (a) Calculate the standard free energy change as a pair of electrons is trans-
ferred from succinate to molecular oxygen in the mitochondrial respira-
tory chain.
(b) Based on your answer in part a, calculate the maximum number of
protons that could be pumped out of the matrix into the intermembrane
space as these electrons are passed to oxygen. Assume 25 °C, ApH = 1.4;
Aựt = 0.175 V (matrix negative).
(c) At which site(s) are these protons pumped?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 7 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning