A certain half-reaction has a standard reduction potential E- =-0.77 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell tha must provide at least 1.30 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell Is there a minimum standard reduction 0 =v yes, there is a minimum. E potential that the half-reaction used at red the anode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box X no minimum Is there a maximum standard reduction 0 E red 3 Пv O yes, there is a maximum potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 mal places. If there is no upper limit, check the "no" box no maximum By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the anode of this cell Note: write the half reaction as it would actually occur at the anode. Ag (aq)e- Ag (s) Al3+ (aq) 3e Al (s) 0.7996 -1.676 Au (aq) e" Au (s) 1.692 Au3+ (aq) 3e Au (s) 1.498 Ba2+ (aq)2e Ba (s) -2.912 Br2 ()2e - 2Br (aq) 1.066 Ca2+ (aq)2e - - Са (s) -2.868 Cl2 (g)2e » 2Cl (aq) 1.35827 Co2+ (aq) 2e Co (s) -0.28 Co3+ (aq) e- Co2+ (aq) 1.92 Cr2+ (aq) 2e Cr (s) -0.913 Cr3+ (ag) 3e Cr (s) -0.744 Cr3+ (aq) e- Cr2+ (aq) -0.407 CrO42- (aq) 4H20 (I) +3e Cr(OH)3 (s) 50H- (aq) -0.13 Cu2+ (aq)2e- Cu (s) 0.3419 Cu2+ (aq) e Cu (aq) 0.153 Cu (aq)e-Cu (s) 0.521 F2 (g)2e 2F (aq) Fe2+ (aq)2e 2.866 Fe (s) -0.447 Fe3+ (aq)e"- Fe2+ (aq) 0.771 Fe3+ (aq) 3e Fe (s) -0.037 2H+ (aq) 2e H2 (g) 0.000 2H20 ()2e- H2 (g) + 20H (aq) -0.8277
A certain half-reaction has a standard reduction potential E- =-0.77 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell tha must provide at least 1.30 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell Is there a minimum standard reduction 0 =v yes, there is a minimum. E potential that the half-reaction used at red the anode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box X no minimum Is there a maximum standard reduction 0 E red 3 Пv O yes, there is a maximum potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 mal places. If there is no upper limit, check the "no" box no maximum By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the anode of this cell Note: write the half reaction as it would actually occur at the anode. Ag (aq)e- Ag (s) Al3+ (aq) 3e Al (s) 0.7996 -1.676 Au (aq) e" Au (s) 1.692 Au3+ (aq) 3e Au (s) 1.498 Ba2+ (aq)2e Ba (s) -2.912 Br2 ()2e - 2Br (aq) 1.066 Ca2+ (aq)2e - - Са (s) -2.868 Cl2 (g)2e » 2Cl (aq) 1.35827 Co2+ (aq) 2e Co (s) -0.28 Co3+ (aq) e- Co2+ (aq) 1.92 Cr2+ (aq) 2e Cr (s) -0.913 Cr3+ (ag) 3e Cr (s) -0.744 Cr3+ (aq) e- Cr2+ (aq) -0.407 CrO42- (aq) 4H20 (I) +3e Cr(OH)3 (s) 50H- (aq) -0.13 Cu2+ (aq)2e- Cu (s) 0.3419 Cu2+ (aq) e Cu (aq) 0.153 Cu (aq)e-Cu (s) 0.521 F2 (g)2e 2F (aq) Fe2+ (aq)2e 2.866 Fe (s) -0.447 Fe3+ (aq)e"- Fe2+ (aq) 0.771 Fe3+ (aq) 3e Fe (s) -0.037 2H+ (aq) 2e H2 (g) 0.000 2H20 ()2e- H2 (g) + 20H (aq) -0.8277
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.4QAP: Halide ions can he deposited at a silver anode, the reaction being Ag(s) + X- AgX(s) +e- Suppose...
Related questions
Question
If you need the rest of the question let me know
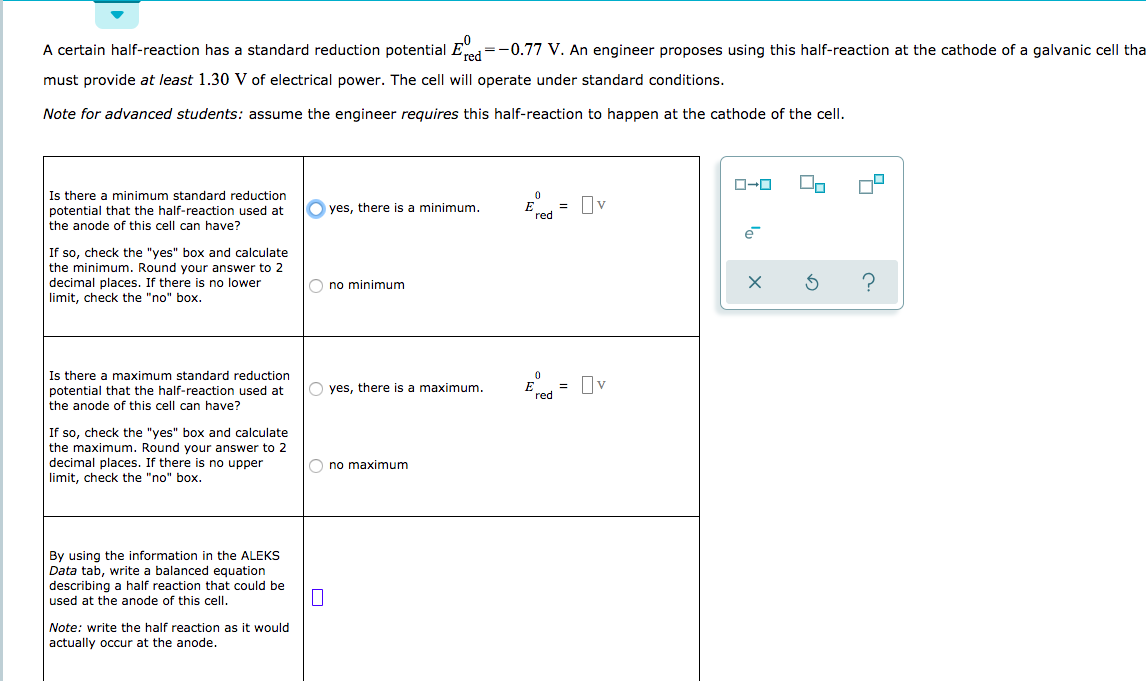
Transcribed Image Text:A certain half-reaction has a standard reduction potential E-
=-0.77 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell tha
must provide at least 1.30 V of electrical power. The cell will operate under standard conditions.
Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell
Is there a minimum standard reduction
0
=v
yes, there is a minimum.
E
potential that the half-reaction used at
red
the anode of this cell can have?
If so, check the "yes" box and calculate
the minimum. Round your answer to 2
decimal places. If there is no lower
limit, check the "no" box
X
no minimum
Is there a maximum standard reduction
0
E
red
3 Пv
O yes, there is a maximum
potential that the half-reaction used at
the anode of this cell can have?
If so, check the "yes" box and calculate
the maximum. Round your answer to 2
mal places. If there is no upper
limit, check the "no" box
no maximum
By using the information in the ALEKS
Data tab, write a balanced equation
describing a half reaction that could be
used at the anode of this cell
Note: write the half reaction as it would
actually occur at the anode.
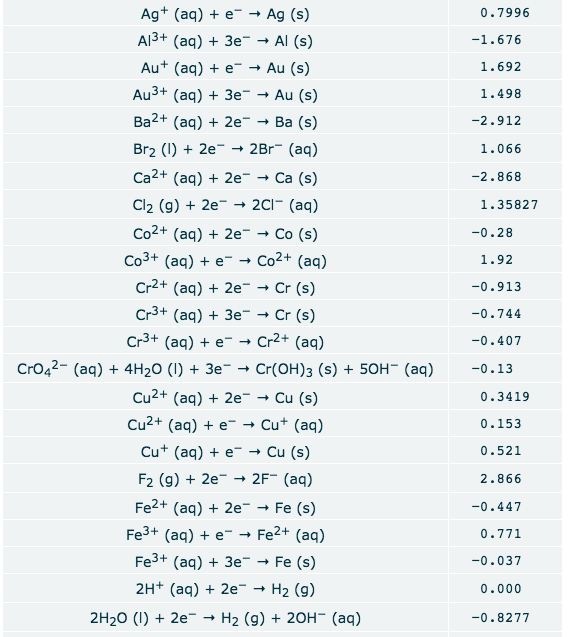
Transcribed Image Text:Ag (aq)e- Ag (s)
Al3+ (aq) 3e Al (s)
0.7996
-1.676
Au (aq) e" Au (s)
1.692
Au3+ (aq) 3e
Au (s)
1.498
Ba2+ (aq)2e
Ba (s)
-2.912
Br2 ()2e - 2Br (aq)
1.066
Ca2+ (aq)2e -
- Са (s)
-2.868
Cl2 (g)2e » 2Cl (aq)
1.35827
Co2+ (aq) 2e
Co (s)
-0.28
Co3+ (aq) e- Co2+ (aq)
1.92
Cr2+ (aq) 2e
Cr (s)
-0.913
Cr3+ (ag) 3e Cr (s)
-0.744
Cr3+ (aq) e- Cr2+ (aq)
-0.407
CrO42- (aq) 4H20 (I) +3e
Cr(OH)3 (s)
50H- (aq)
-0.13
Cu2+ (aq)2e-
Cu (s)
0.3419
Cu2+ (aq) e Cu (aq)
0.153
Cu (aq)e-Cu (s)
0.521
F2 (g)2e 2F (aq)
Fe2+ (aq)2e
2.866
Fe (s)
-0.447
Fe3+ (aq)e"- Fe2+ (aq)
0.771
Fe3+ (aq) 3e
Fe (s)
-0.037
2H+ (aq) 2e
H2 (g)
0.000
2H20 ()2e- H2 (g) + 20H (aq)
-0.8277
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning