A certain metal M forms a soluble sulfate salt M₂(SO4)3. Suppose the left half cell of a galvanic cell apparatus is filled with a 4.50 M solution of M₂(SO4)3 and the right half cell with a 450. mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 40.0 °C. Which electrode will be positive? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. O left O right 0 x10 X
A certain metal M forms a soluble sulfate salt M₂(SO4)3. Suppose the left half cell of a galvanic cell apparatus is filled with a 4.50 M solution of M₂(SO4)3 and the right half cell with a 450. mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 40.0 °C. Which electrode will be positive? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. O left O right 0 x10 X
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 92QRT
Related questions
Question
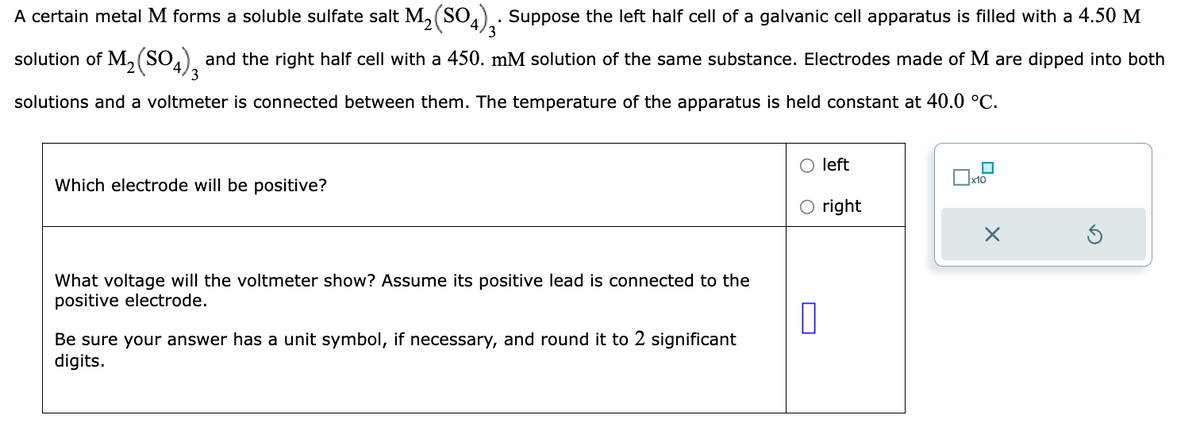
Transcribed Image Text:A certain metal M forms a soluble sulfate salt M₂(SO4)3. Suppose the left half cell of a galvanic cell apparatus is filled with a 4.50 M
solution of M₁₂(SO4), and the right half cell with a 450. mM solution of the same substance. Electrodes made of M are dipped into both
3
solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 40.0 °C.
Which electrode will be positive?
What voltage will the voltmeter show? Assume its positive lead is connected to the
positive electrode.
Be sure your answer has a unit symbol, if necessary, and round it to 2 significant
digits.
П
left
right
☐
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning