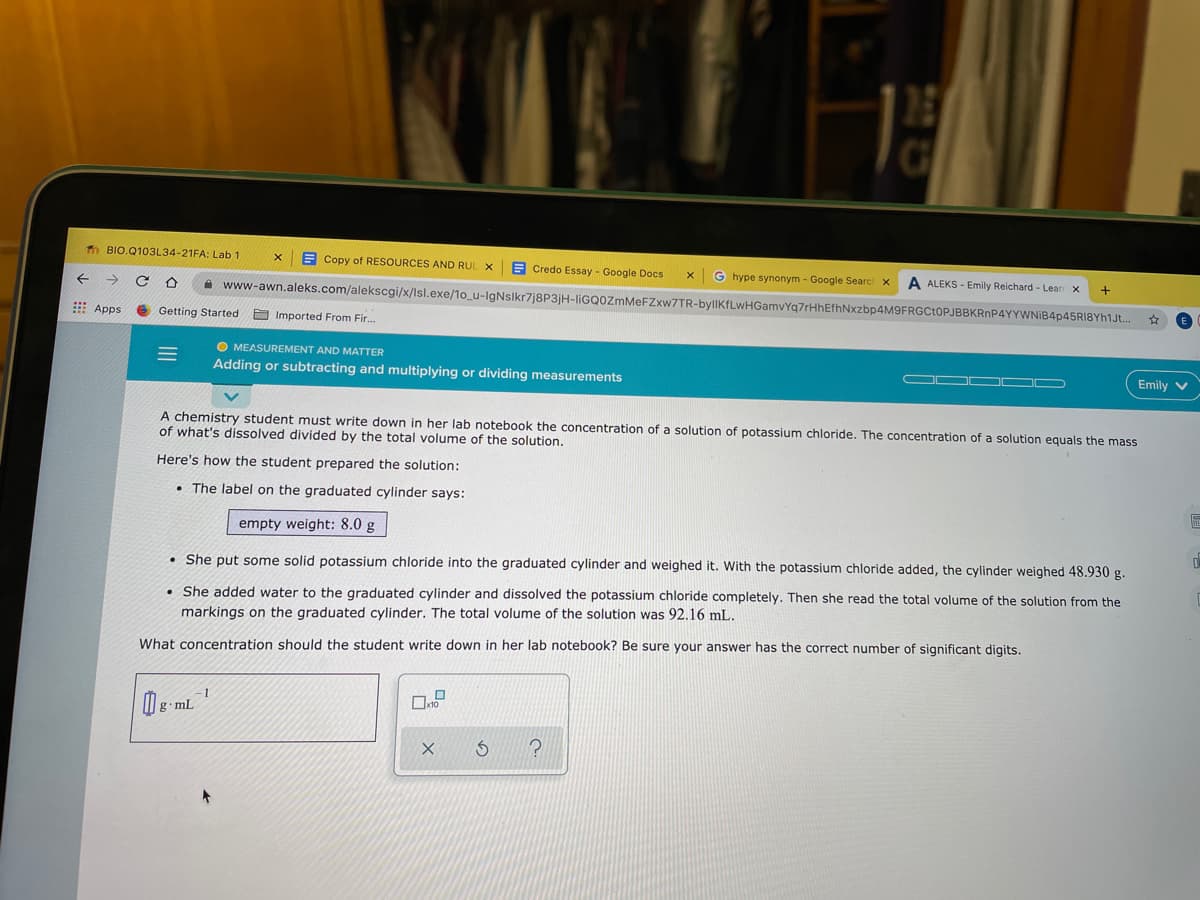
A chemistry student must write down in her lab notebook the concentration of a solution of potassium chloride. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 8.0 g • She put some solid potassium chloride into the graduated cylinder and weighed it. With the potassium chloride added, the cylinder weighed 48.930 g. • She added water to the graduated cylinder and dissolved the potassium chloride completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 92.16 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits. -1
A chemistry student must write down in her lab notebook the concentration of a solution of potassium chloride. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 8.0 g • She put some solid potassium chloride into the graduated cylinder and weighed it. With the potassium chloride added, the cylinder weighed 48.930 g. • She added water to the graduated cylinder and dissolved the potassium chloride completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 92.16 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits. -1
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.21P
Related questions
Question
Transcribed Image Text:o BIO.0103L34-21FA: Lab 1
E Copy of RESOURCES AND RUL X
E Credo Essay - Google Docs
G hype synonym - Google Searc x
A ALEKS - Emily Reichard - Lear
A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IIGQOZMMEFZXW7TR-bylIKfLwHGamvYq7rHhEfhNxzbp4M9FRGCtOPJBBKRnP4YYWNIB4p45RI8Yh1Jt.
E Apps
6 Getting Started
E Imported From Fir.
O MEASUREMENT AND MATTER
三
Adding or subtracting and multiplying or dividing measurements
OO OD D
Emily v
A chemistry student must write down in her lab notebook the concentration of a solution of potassium chloride. The concentration of a solution equals the mass
of what's dissolved divided by the total volume of the solution.
Here's how the student prepared the solution:
• The label on the graduated cylinder says:
empty weight: 8.0 g
• She put some solid potassium chloride into the graduated cylinder and weighed it. With the potassium chloride added, the cylinder weighed 48.930 g.
• She added water to the graduated cylinder and dissolved the potassium chloride completely. Then she read the total volume of the solution from the
markings on the graduated cylinder. The total volume of the solution was 92.16 mL.
What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits.
-1
g.mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning