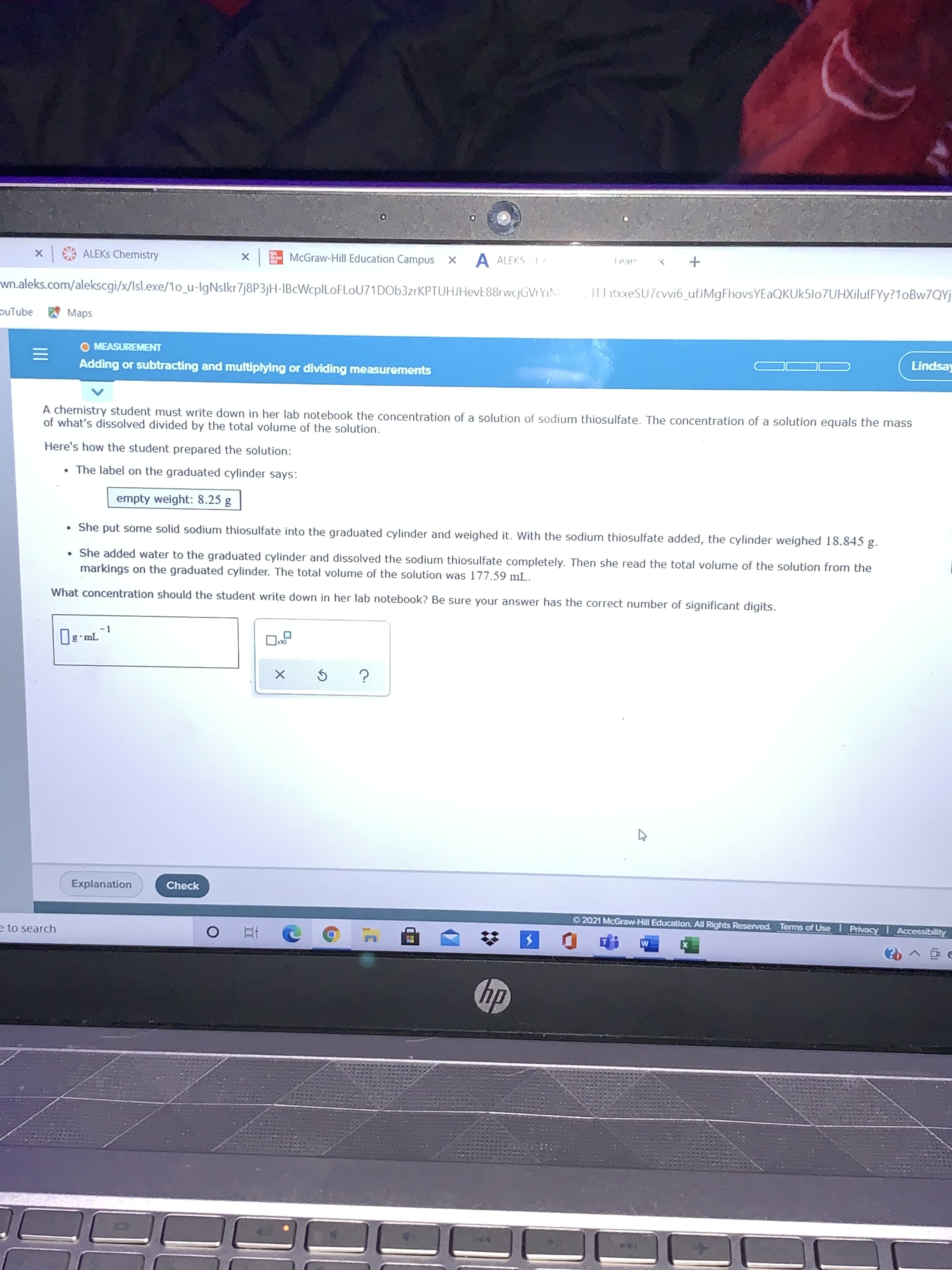
A chemistry student must write down in her lab notebook the concentration of a solution of sodium thiosulfate. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 8.25 g • She put some solid sodium thiosulfate into the graduated cylinder and weighed it. With the sodium thiosulfate added, the cylinder weighed 18.845 g. • She added water to the graduated cylinder and dissolved the sodium thiosulfate completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 177.59 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits.
A chemistry student must write down in her lab notebook the concentration of a solution of sodium thiosulfate. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 8.25 g • She put some solid sodium thiosulfate into the graduated cylinder and weighed it. With the sodium thiosulfate added, the cylinder weighed 18.845 g. • She added water to the graduated cylinder and dissolved the sodium thiosulfate completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 177.59 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
Transcribed Image Text:A chemistry student must write down in her lab notebook the concentration of a solution of sodium thiosulfate. The concentration of a solution equals the mass
of what's dissolved divided by the total volume of the solution.
Here's how the student prepared the solution:
• The label on the graduated cylinder says:
empty weight: 8.25 g
• She put some solid sodium thiosulfate into the graduated cylinder and weighed it. With the sodium thiosulfate added, the cylinder weighed 18.845 g.
• She added water to the graduated cylinder and dissolved the sodium thiosulfate completely. Then she read the total volume of the solution from the
markings on the graduated cylinder. The total volume of the solution was 177.59 mL.
What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning