A chemistry student needs 85.0 g of carbon tetrachloride for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers -3 Calculate the volume of carbon tetrachloride the student should pour out. that the density of carbon tetrachloride is 1.59 g cm Round your answer to 3 significant digits. mL x10 I Don't Know Submit Privacy Terms of Use © 2020 McGraw-Hill Education. All Rights Reserved. Ctv 10 MacBook Pro & 4 Y Q H. option command command tion 00 LL
A chemistry student needs 85.0 g of carbon tetrachloride for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers -3 Calculate the volume of carbon tetrachloride the student should pour out. that the density of carbon tetrachloride is 1.59 g cm Round your answer to 3 significant digits. mL x10 I Don't Know Submit Privacy Terms of Use © 2020 McGraw-Hill Education. All Rights Reserved. Ctv 10 MacBook Pro & 4 Y Q H. option command command tion 00 LL
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter1: Essential Ideas
Section: Chapter Questions
Problem 59E: The label on a soft drink boule gives the volume in two units: 2.0 L and 67.6 fl oz. Use this...
Related questions
Question
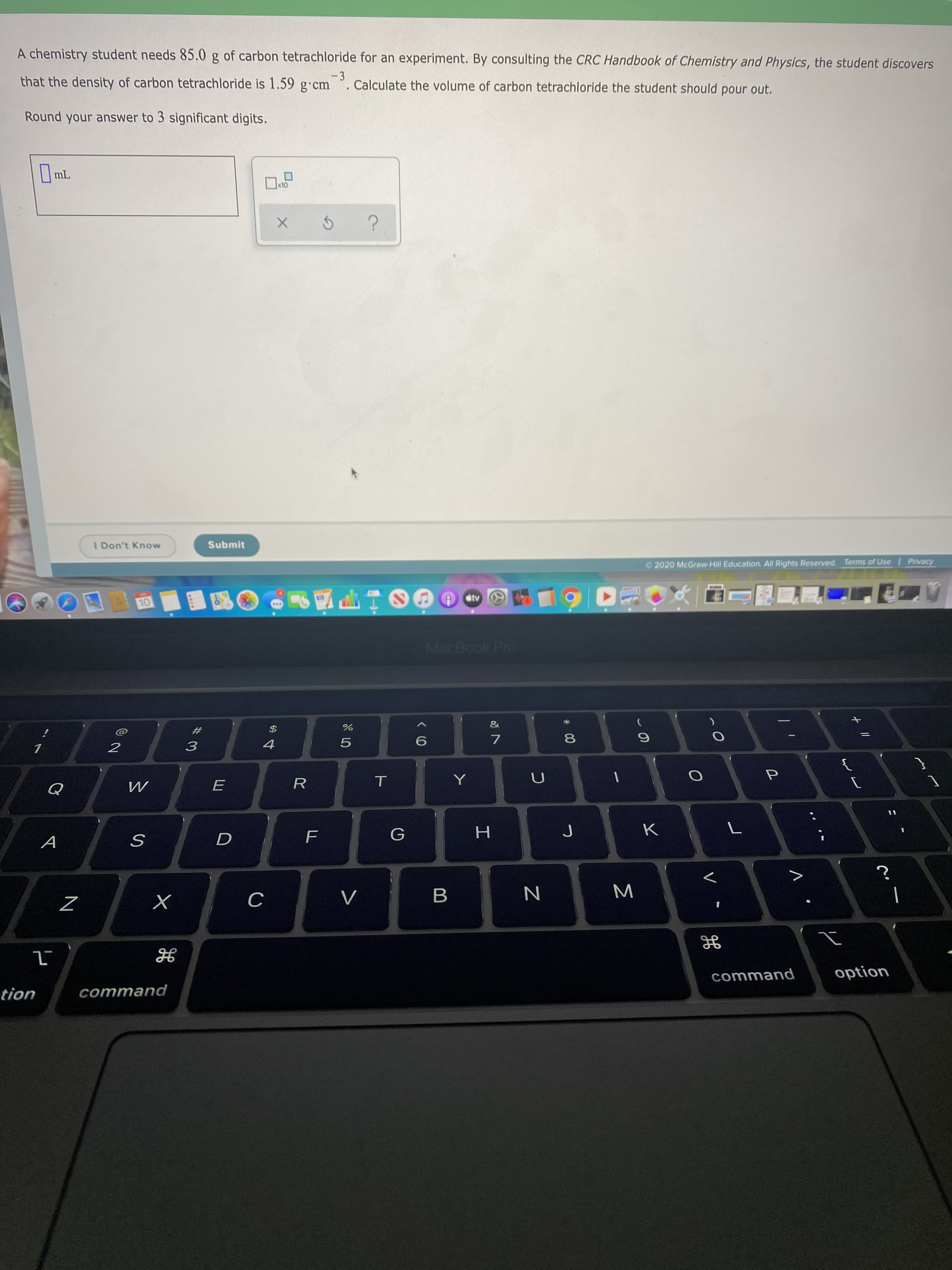
Transcribed Image Text:A chemistry student needs 85.0 g of carbon tetrachloride for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers
-3
Calculate the volume of carbon tetrachloride the student should pour out.
that the density of carbon tetrachloride is 1.59 g cm
Round your answer to 3 significant digits.
mL
x10
I Don't Know
Submit
Privacy
Terms of Use
© 2020 McGraw-Hill Education. All Rights Reserved.
Ctv
10
MacBook Pro
&
4
Y
Q
H.
option
command
command
tion
00
LL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning