A compound is 54.53% C, 9.15% H, and 36.32% O by mass. What is its empirical formula? Insert subscripts as needed. empirical formula: СНО The molecular mass of the compound is 132 amu. What is its molecular formula? Insert subscripts as needed. molecular formula: СНО
A compound is 54.53% C, 9.15% H, and 36.32% O by mass. What is its empirical formula? Insert subscripts as needed. empirical formula: СНО The molecular mass of the compound is 132 amu. What is its molecular formula? Insert subscripts as needed. molecular formula: СНО
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section3.9: Composition And Empirical Formulas
Problem 3.21CE: Phenol is a compound of carbon, hydrogen, and oxygen used commonly as a disinfectant. Combustion of...
Related questions
Question
Need help with empirical and molecular formulas
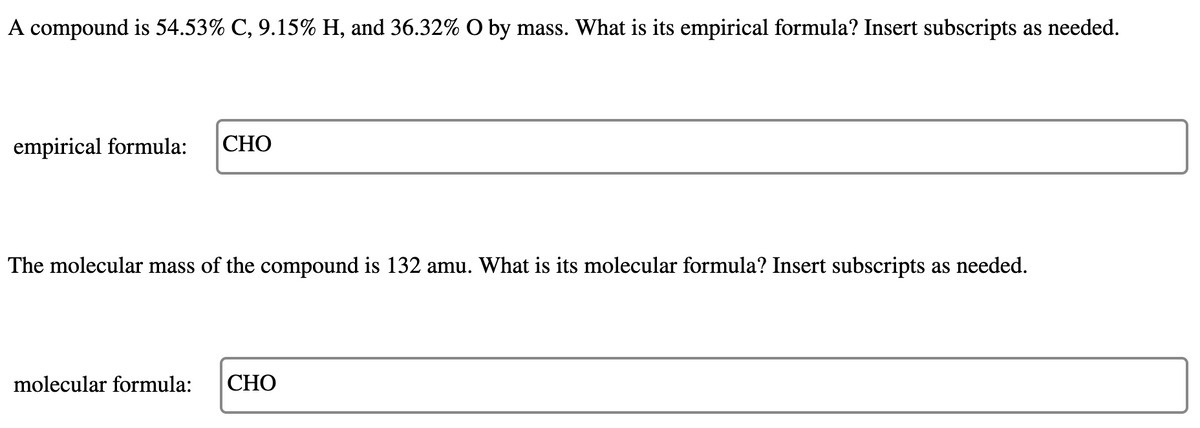
Transcribed Image Text:A compound is 54.53% C, 9.15% H, and 36.32% O by mass. What is its empirical formula? Insert subscripts as needed.
empirical formula:
СНО
The molecular mass of the compound is 132 amu. What is its molecular formula? Insert subscripts as needed.
molecular formula:
СНО
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning