Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 21ALQ: True or false? The atom with the largest subscript in a formula is the atom with the largest percent...
Related questions
Question
![[Review Topics]
[References]
Use the References to access important values if needed for this question.
A compound is found to contain 64.80 % carbon, 13.62 % hydrogen, and 21.58 % oxygen by mass.
What is the empirical formula for this compound?
To answer the question, enter the elements in the order presented above.
Submit Answer
Retry Entire Group
3 more group attempts remaining
Cengage Learning | Cengage Technical Support
Q Search Bing
$
7
8.
5
CO](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F728daa52-c1e6-4aac-ae8f-ab0c6ea6f8ed%2F81d22040-a2ca-4076-9640-710cfcf4d2b5%2Fr60i289_processed.jpeg&w=3840&q=75)
Transcribed Image Text:[Review Topics]
[References]
Use the References to access important values if needed for this question.
A compound is found to contain 64.80 % carbon, 13.62 % hydrogen, and 21.58 % oxygen by mass.
What is the empirical formula for this compound?
To answer the question, enter the elements in the order presented above.
Submit Answer
Retry Entire Group
3 more group attempts remaining
Cengage Learning | Cengage Technical Support
Q Search Bing
$
7
8.
5
CO
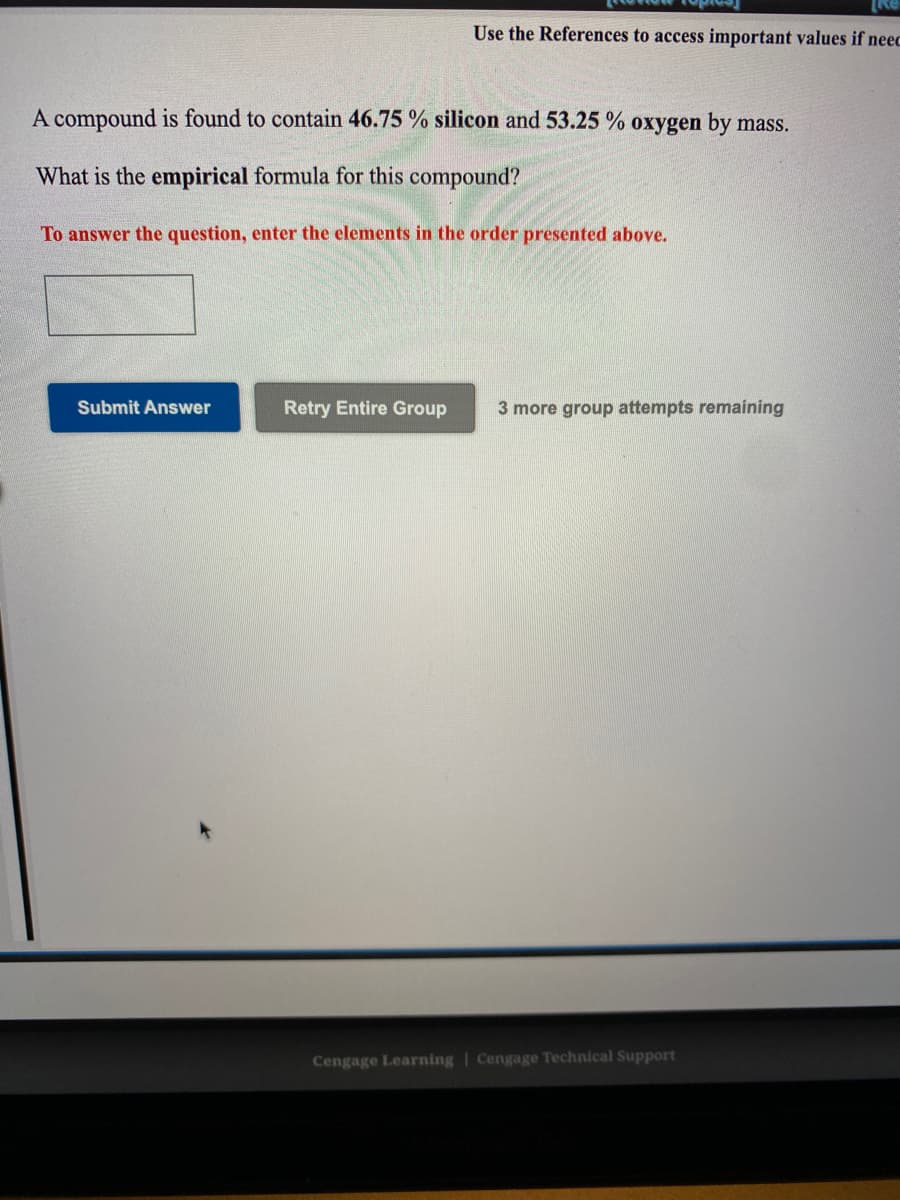
Transcribed Image Text:Use the References to access important values if neec
A compound is found to contain 46.75 % silicon and 53.25 % oxygen by mass.
What is the empirical formula for this compound?
To answer the question, enter the elements in the order presented above.
Submit Answer
Retry Entire Group
3 more group attempts remaining
Cengage Learning | Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co